Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria
- PMID: 15273119
- PMCID: PMC478526
- DOI: 10.1128/AAC.48.8.3043-3050.2004
Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria
Abstract
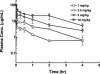
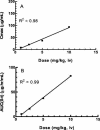
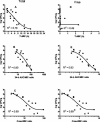
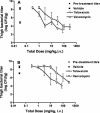
Telavancin (TD-6424) is a novel lipoglycopeptide that produces rapid and concentration-dependent killing of clinically relevant gram-positive organisms in vitro. The present studies evaluated the in vivo pharmacodynamics of telavancin in the mouse neutropenic thigh (MNT) and mouse subcutaneous infection (MSI) animal models. Pharmacokinetic-pharmacodynamic studies in the MNT model demonstrated that the 24-h area under the concentration-time curve (AUC)/MIC ratio was the best predictor of efficacy. Telavancin produced dose-dependent reduction of thigh titers of several organisms, including methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA), penicillin-susceptible and -resistant strains of Streptococcus pneumoniae, and vancomycin-resistant Enterococcus faecalis. The 50% effective dose (ED50) estimates for telavancin ranged from 0.5 to 6.6 mg/kg of body weight (administered intravenously), and titers were reduced by up to 3 log10 CFU/g from pretreatment values. Against MRSA ATCC 33591, telavancin was 4- and 30-fold more potent (on an ED50 basis) than vancomycin and linezolid, respectively. Against MSSA ATCC 13709, telavancin was 16- and 40-fold more potent than vancomycin and nafcillin, respectively. Telavancin, vancomycin, and linezolid were all efficacious and more potent against MRSA ATCC 33591 in the MSI model compared to the MNT model. This deviation in potency was, however, disproportionately greater for vancomycin and linezolid than for telavancin, suggesting that activity of telavancin is less affected by the immune status. The findings of these studies collectively suggest that once-daily dosing of telavancin may provide an effective approach for the treatment of clinically relevant infections with gram-positive organisms.
Figures

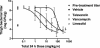
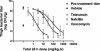
References
-
- Andes, D., and W. A. Craig. 2001. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. - PubMed
-
- CDC NNIS System. 2001. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. - PubMed
-
- Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin-United States, 2002. Morbid. Mortal. Wkly. Rep. 51:565-567. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic paramaters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. - PubMed
-
- Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

