Antigen transport and cytoskeletal characteristics of a distinct enterocyte population in inflammatory bowel diseases
- PMID: 15277217
- PMCID: PMC1618561
- DOI: 10.1016/S0002-9440(10)63308-1
Antigen transport and cytoskeletal characteristics of a distinct enterocyte population in inflammatory bowel diseases
Abstract
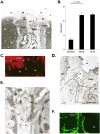
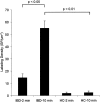
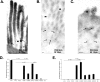
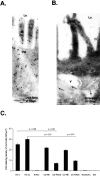
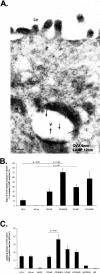
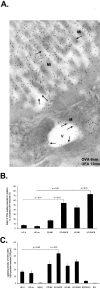
Intestinal antigen uptake is enhanced in inflammatory bowel disease. We analyzed transcellular transport routes of antigens in different compartments of normal enterocytes and atypical intestinal epithelial cells called "rapid antigen uptake into the cytosol enterocytes" (RACE cells). These cells constitute a recently described population of enterocyte-derived cells, which are increased in inflammatory bowel disease. Mucosa of freshly resected specimens were incubated with the antigens ovalbumin or horseradish peroxidase. Ultrastructural labeling patterns of differentiation-dependent proteins, the brush-border enzyme sucrase-isomaltase and the cytoskeleton proteins villin and actin, were determined in enterocytes. Apoptosis was investigated biochemically and ultrastructurally by cleavage of caspase-3. Both antigens were transported to late endosomes and to trans-Golgi vesicles of enterocytes in inflammatory bowel disease and control specimens. Quantitative evaluation revealed a significantly increased transepithelial antigen transport in both compartments of RACE relative to normal enterocytes. Labeling densities for sucrase-isomaltase, villin, and actin were decreased in RACE relative to normal enterocytes. Caspase-3 was not increased in RACE cells relative to controls. RACE cells are characterized by increased antigen transport to late endosomes and the trans-Golgi network, a disassembled cytoskeleton and lower concentrations of proteins that are markers of cell differentiation.
Figures


References
-
- Dvorak AM, Dickersin GR. Crohn’s disease: transmission electron microscopic studies. I. Barrier function. Possible changes related to alterations of cell coat, mucous coat, epithelial cells, and Paneth cells. Hum Pathol. 1980;11:561–571. - PubMed
-
- Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. - PubMed
-
- Soderholm JD, Peterson KH, Olaison G, Franzen LE, Westrom B, Magnusson KE, Sjodahl R. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology. 1999;117:65–72. - PubMed
-
- Schurmann G, Bruwer M, Klotz A, Schmid KW, Senninger N, Zimmer KP. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis. 1999;14:41–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

