Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer's disease
- PMID: 15277226
- PMCID: PMC1618555
- DOI: 10.1016/S0002-9440(10)63317-2
Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer's disease
Abstract
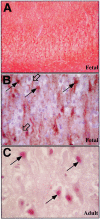
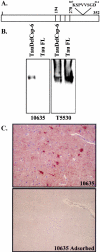
Previously, we have shown that caspase-6 but not caspase-3 is activated by serum deprivation and induces a protracted cell death in primary cultures of human neurons (LeBlanc AC, Liu H, Goodyer C, Bergeron C, Hammond J: Caspase-6 role in apoptosis of human neurons, amyloidogenesis and Alzheimer's disease. J Biol Chem 1999, 274:23426-23436 and Zhang Y, Goodyer C, LeBlanc A: Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci 2000, 20:8384-8389). Here, we show with neoepitope antibodies that the p20 subunit of active caspase-6 increases twofold to threefold in the affected temporal and frontal cortex but not in the unaffected cerebellum of Alzheimer's disease brains and is present in neurofibrillary tangles, neuropil threads, and the neuritic plaques. Furthermore, a neoepitope antibody to caspase-6-cleaved Tau strongly detects intracellular tangles, extracellular tangles, pretangles, neuropil threads, and neuritic plaques. Immunoreactivity with both antibodies in pretangles indicates that the caspase-6 is active early in the pathogenesis of Alzheimer's disease. In contrast to the nuclear and cytosolic localization of active caspase-6 in apoptotic neurons of fetal and adult ischemic brains, the active caspase-6 in Alzheimer's disease brains is sequestered into the tangles or neurites. The localization of active caspase-6 may strongly jeopardize the structural integrity of the neuronal cytoskeletal system leading to inescapable neuronal dysfunction and eventual cell death in Alzheimer's disease neurons. Our results suggest that active caspase-6 is strongly implicated in human neuronal degeneration and apoptosis.
Figures





References
-
- LeBlanc AC, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis and Alzheimer’s Disease. J Biol Chem. 1999;274:23426–23436. - PubMed
-
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. - PubMed
-
- Gervais F, Xu D, Robertson G, Vaillancourt J, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman M, Clarke E, Zheng H, Van Der Ploeg L, Ruffolo S, Thornberry N, Xanthoudakis S, Zamboni R, Roy S, Nicholson D. Involvement of caspases in proteolytic cleavage of Alzheimer’s β-amyloid precursor protein and amyloidogenic β-peptide formation. Cell. 1999;97:395–406. - PubMed
-
- Weidemann A, Paliga K, Durrwang U, Reinhard F, Zchuckert O, Evin G, Masters CL. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like enzymes. J Biol Chem. 1999;274:5823–5829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

