The 14-3-3 protein epsilon isoform expressed in reactive astrocytes in demyelinating lesions of multiple sclerosis binds to vimentin and glial fibrillary acidic protein in cultured human astrocytes
- PMID: 15277231
- PMCID: PMC1618573
- DOI: 10.1016/s0002-9440(10)63322-6
The 14-3-3 protein epsilon isoform expressed in reactive astrocytes in demyelinating lesions of multiple sclerosis binds to vimentin and glial fibrillary acidic protein in cultured human astrocytes
Abstract
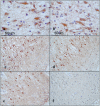
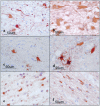
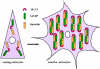
The 14-3-3 protein family consists of acidic 30-kd proteins expressed at high levels in neurons of the central nervous system. Seven isoforms form a dimeric complex that acts as a molecular chaperone that interacts with key signaling components. Recent studies indicated that the 14-3-3 protein identified in the cerebrospinal fluid of various neurological diseases including multiple sclerosis (MS) is a marker for extensive brain destruction. However, it remains unknown whether the 14-3-3 protein plays an active role in the pathological process of MS. To investigate the differential expression of seven 14-3-3 isoforms in MS lesions, brain tissues of four progressive cases were immunolabeled with a panel of isoform-specific antibodies. Reactive astrocytes in chronic demyelinating lesions intensely expressed beta, epsilon, zeta, eta, and sigma isoforms, among which the epsilon isoform is a highly specific marker for reactive astrocytes. Furthermore, protein overlay, mass spectrometry, immunoprecipitation, and double-immunolabeling analysis showed that the 14-3-3 protein interacts with both vimentin and glial fibrillary acidic protein in cultured human astrocytes. These results suggest that the 14-3-3 protein plays an organizing role in the intermediate filament network in reactive astrocytes at the site of demyelinating lesions in MS.
Figures








Similar articles
-
Vimentin-positive astrocytes in canine distemper: a target for canine distemper virus especially in chronic demyelinating lesions?Acta Neuropathol. 2007 Dec;114(6):597-608. doi: 10.1007/s00401-007-0307-5. Epub 2007 Oct 27. Acta Neuropathol. 2007. PMID: 17965866
-
The effect of ribavirin on reactive astrogliosis in experimental autoimmune encephalomyelitis.J Pharmacol Sci. 2012;119(3):221-32. doi: 10.1254/jphs.12004fp. Epub 2012 Jun 28. J Pharmacol Sci. 2012. PMID: 22785017
-
Increased 14-3-3 immunoreactivity in glial elements in patients with multiple sclerosis.Acta Neuropathol. 2004 Feb;107(2):137-43. doi: 10.1007/s00401-003-0785-z. Epub 2003 Nov 6. Acta Neuropathol. 2004. PMID: 14605832
-
Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system.Curr Opin Cell Biol. 2015 Feb;32:121-30. doi: 10.1016/j.ceb.2015.02.004. Epub 2015 Mar 2. Curr Opin Cell Biol. 2015. PMID: 25726916 Review.
-
Association between cell-mediated demyelination and astrocyte stimulation.Prog Brain Res. 1992;94:411-22. doi: 10.1016/s0079-6123(08)61768-9. Prog Brain Res. 1992. PMID: 1287726 Review. No abstract available.
Cited by
-
Are cerebrospinal fluid biomarkers useful in predicting the prognosis of multiple sclerosis patients?Int J Mol Sci. 2011;12(11):7960-70. doi: 10.3390/ijms12117960. Epub 2011 Nov 16. Int J Mol Sci. 2011. PMID: 22174643 Free PMC article. Review.
-
Microwave and magnetic (M(2) ) proteomics of the experimental autoimmune encephalomyelitis animal model of multiple sclerosis.Electrophoresis. 2012 Dec;33(24):3810-9. doi: 10.1002/elps.201200200. Electrophoresis. 2012. PMID: 23161666 Free PMC article.
-
Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties.Genes Nutr. 2009 Dec;4(4):283-96. doi: 10.1007/s12263-009-0143-4. Epub 2009 Sep 10. Genes Nutr. 2009. PMID: 19756809 Free PMC article.
-
Proteomic analysis of human cerebral endothelial cells activated by multiple sclerosis serum and IFNbeta-1b.J Mol Neurosci. 2007;32(3):169-78. doi: 10.1007/s12031-007-0018-3. J Mol Neurosci. 2007. PMID: 17873362
-
Neuroprotective function of 14-3-3 proteins in neurodegeneration.Biomed Res Int. 2013;2013:564534. doi: 10.1155/2013/564534. Epub 2013 Dec 2. Biomed Res Int. 2013. PMID: 24364034 Free PMC article. Review.
References
-
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. - PubMed
-
- van Hemert MJ, Steensma HY, van Heusden GPH. 14-3-3 proteins: key regulators of cell division, signaling and apoptosis. Bioessays. 2001;23:936–947. - PubMed
-
- Aitken A, Baxter H, Dubois T, Clokie S, Mackie S, Mitchell K, Peden A, Zemlickova E. 14-3-3 proteins in cell regulation. Biochem Soc Trans. 2002;30:351–360. - PubMed
-
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nature Rev Neurosci. 2002;4:752–762. - PubMed
-
- Boston PF, Jackson P, Kyonoch PAM, Thompson RJ. Purification, properties, and immunohistochemical localisation of human brain 14-3-3 protein. J Neurochem. 1982;38:1466–1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

