Cathepsin K is the principal protease in giant cell tumor of bone
- PMID: 15277232
- PMCID: PMC1618565
- DOI: 10.1016/S0002-9440(10)63323-8
Cathepsin K is the principal protease in giant cell tumor of bone
Abstract
Giant cell tumor (GCT) of bone is a neoplasm of bone characterized by a localized osteolytic lesion. The nature of GCT is an enigma and the cell type(s) and protease(s) responsible for the extensive localized clinicoradiological osteolysis remain unresolved. We evaluated protease expression and cellular distribution of the proteolytic machinery responsible for the osteolysis. mRNA profiles showed that cathepsin K, cathepsin L, and matrix metalloproteinase (MMP)-9 were the preferentially expressed collagenases. Moderate expression was found for MMP-13, MMP-14, and cathepsin S. Specific protease activity assays revealed high cathepsin K activity but showed that MMP-9 was primarily present (98%) as inactive proenzyme. Activities of MMP-13 and MMP-14 were low. Immunohistochemistry revealed a clear spatial distribution: cathepsin K, its associated proton pump V-H(+)-ATPase, and MMP-9 were exclusively expressed in osteoclast-like giant cells, whereas cathepsin L expression was confined to mononuclear cells. To explore a possible role of cathepsin L in osteolysis, GCT-derived, cathepsin L-expressing, mononuclear cells were cultured on dentine disks. No evidence of osteolysis by these cells was found. These results implicate cathepsin K as the principal protease in GCT and suggest that osteoclast-like giant cells are responsible for the osteolysis. Inhibition of cathepsin K or its associated proton-pump may provide new therapeutic opportunities for GCT.
Figures

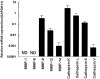


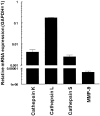

References
-
- Reid R, Banerjee SS, Sciot R. Giant cell tumour. Fletcher CDM, Unni KK, Mertens F, editors. Lyon: IARC Press,; World Health Organization Classification of Tumours. Pathology and Genetics, Tumours of Soft Tissue and Bone. 2002:pp 310–312.
-
- Wülling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001;127:467–474. - PubMed
-
- Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM. Giant cell tumor of the long bone: a Canadian sarcoma group study. Clin Orthop. 2002;397:248–258. - PubMed
-
- Mills BG, Frausto A. Cytokines expressed in multinucleated cells: Paget’s disease and giant cell tumors versus normal bone. Calcif Tissue Int. 1997;61:16–21. - PubMed
-
- Zheng MH, Robbins P, Xu J, Huang L, Wood DJ, Papadimitriou JM. The histogenesis of giant cell tumour of bone: a model of interaction between neoplastic cells and osteoclasts. Histol Histopathol. 2001;16:297–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

