Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury
- PMID: 15277235
- PMCID: PMC1618576
- DOI: 10.1016/s0002-9440(10)63326-3
Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury
Abstract
Severity of fibrosis after injury is determined by the nature of the injury and host genetic susceptibility. Metabolism of collagen, the major component of fibrotic lesions, is, in part, regulated by integrins. Using a model of glomerular injury by adriamycin, which induces reactive oxygen species (ROS) production, we demonstrated that integrin alpha1-null mice develop more severe glomerulosclerosis than wild-type mice. Moreover, primary alpha1-null mesangial cells produce more ROS both at baseline and after adriamycin treatment. Increased ROS synthesis leads to decreased cell proliferation and increased glomerular collagen IV accumulation that is reversed by antioxidants both in vivo and in vitro. Thus, we have identified integrin alpha1beta1 as a modulator of glomerulosclerosis. In addition, we showed a novel pathway where integrin alpha1beta1 modulates ROS production, which in turn controls collagen turnover and ultimately fibrosis. Because integrin alpha1beta1 is expressed in many cell types this may represent a generalized mechanism of controlling matrix accumulation, which has implications for numerous diseases characterized by fibrosis.
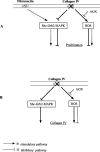
Figures
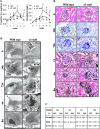
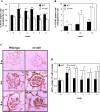
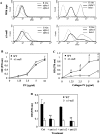
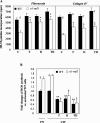



Similar articles
-
Glomerular injury is exacerbated in diabetic integrin alpha1-null mice.Kidney Int. 2006 Aug;70(3):460-70. doi: 10.1038/sj.ki.5000359. Epub 2006 Jun 14. Kidney Int. 2006. PMID: 16775606
-
Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation.Mol Cell Biol. 2007 May;27(9):3313-26. doi: 10.1128/MCB.01476-06. Epub 2007 Mar 5. Mol Cell Biol. 2007. PMID: 17339338 Free PMC article.
-
Overexpression of alpha1beta1 integrin directly affects rat mesangial cell behavior.Kidney Int. 2000 Sep;58(3):1088-97. doi: 10.1046/j.1523-1755.2000.00266.x. Kidney Int. 2000. PMID: 10972673
-
[Glomerular extracellular matrix in glomerulosclerosis by molecular biology].Nihon Rinsho. 1992 Dec;50(12):3038-45. Nihon Rinsho. 1992. PMID: 1491457 Review. Japanese.
-
Synthetic heterotrimeric collagen peptides as mimics of cell adhesion sites of the basement membrane.Biopolymers. 2004;76(1):34-47. doi: 10.1002/bip.10569. Biopolymers. 2004. PMID: 14997473 Review.
Cited by
-
Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression.Mol Cell Biol. 2010 Jun;30(12):3048-58. doi: 10.1128/MCB.00892-09. Epub 2010 Apr 5. Mol Cell Biol. 2010. PMID: 20368353 Free PMC article.
-
Integrins, CAFs and Mechanical Forces in the Progression of Cancer.Cancers (Basel). 2019 May 24;11(5):721. doi: 10.3390/cancers11050721. Cancers (Basel). 2019. PMID: 31137693 Free PMC article. Review.
-
Stimulation of Fibronectin Matrix Assembly by Lysine Acetylation.Cells. 2020 Mar 8;9(3):655. doi: 10.3390/cells9030655. Cells. 2020. PMID: 32182705 Free PMC article.
-
β1 Integrin regulates adult lung alveolar epithelial cell inflammation.JCI Insight. 2020 Jan 30;5(2):e129259. doi: 10.1172/jci.insight.129259. JCI Insight. 2020. PMID: 31873073 Free PMC article.
-
Brief report: enrichment of associations in genes with fibrosis, apoptosis, and innate immunity functions with cardiac manifestations of neonatal lupus.Arthritis Rheum. 2012 Dec;64(12):4060-5. doi: 10.1002/art.34663. Arthritis Rheum. 2012. PMID: 22886516 Free PMC article.
References
-
- Fogo AB. Mesangial matrix modulation and glomerulosclerosis. Exp Nephrol. 1999;7:147–159. - PubMed
-
- Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Voigt S, Gossrau R, Baum O, Loster K, Hofmann W, Reutter W. Distribution and quantification of alpha 1-integrin subunit in rat organs. Histochem J. 1995;27:123–132. - PubMed
-
- Korhonen M, Ylanne J, Laitinen L, Virtanen I. Distribution of beta 1 and beta 3 integrins in human fetal and adult kidney. Lab Invest. 1990;62:616–625. - PubMed
-
- Shikata K, Makino H, Morioka S, Kashitani T, Hirata K, Ota Z, Wada J, Kanwar YS. Distribution of extracellular matrix receptors in various forms of glomerulonephritis. Am J Kidney Dis. 1995;25:680–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK48831/DK/NIDDK NIH HHS/United States
- R01 CA94849-01/CA/NCI NIH HHS/United States
- R01 CA094849/CA/NCI NIH HHS/United States
- P50 DK039261/DK/NIDDK NIH HHS/United States
- GM15431/GM/NIGMS NIH HHS/United States
- P50 DK044757/DK/NIDDK NIH HHS/United States
- P50 DK39261-16/DK/NIDDK NIH HHS/United States
- R01 DK048831/DK/NIDDK NIH HHS/United States
- K08 DK059975/DK/NIDDK NIH HHS/United States
- P01 CA077839/CA/NCI NIH HHS/United States
- DK59975/DK/NIDDK NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- P50 GM015431/GM/NIGMS NIH HHS/United States
- DK56942/DK/NIDDK NIH HHS/United States
- P50 DK44757/DK/NIDDK NIH HHS/United States
- R01 DK056942/DK/NIDDK NIH HHS/United States
- CA77839/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

