Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases
- PMID: 15277241
- PMCID: PMC1618558
- DOI: 10.1016/S0002-9440(10)63332-9
Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases
Abstract
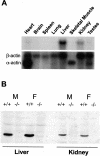
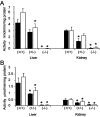
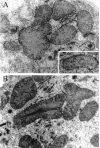
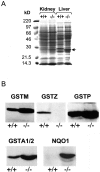
Glutathione transferase zeta (GSTZ1-1) is the major enzyme that catalyzes the metabolism of alpha-halo acids such as dichloroacetic acid, a carcinogenic contaminant of chlorinated water. GSTZ1-1 is identical with maleylacetoacetate isomerase, which catalyzes the penultimate step in the catabolic pathways for phenylalanine and tyrosine. In this study we have deleted the Gstz1 gene in BALB/c mice and characterized their phenotype. Gstz1(-/-) mice do not have demonstrable activity with maleylacetone and alpha-halo acid substrates, and other GSTs do not compensate for the loss of this enzyme. When fed a standard diet, the GSTZ1-1-deficient mice showed enlarged liver and kidneys as well as splenic atrophy. Light and electron microscopic examination revealed multifocal hepatitis and ultrastructural changes in the kidney. The addition of 3% (w/v) phenylalanine to the drinking water was lethal for young mice (<28 days old) and caused liver necrosis, macrovesicular steatosis, splenic atrophy, and a significant loss of circulating leukocytes in older surviving mice. GSTZ1-1-deficient mice showed constitutive induction of alpha, mu, and pi class GSTs as well as NAD(P)H:quinone oxidoreductase 1. The overall response is consistent with the chronic accumulation of a toxic metabolite(s). We detected the accumulation of succinylacetone in the serum of deficient mice but cannot exclude the possibility that maleylacetoacetate and maleylacetone may also accumulate.
Figures





Similar articles
-
Exposure of Rats to Multiple Oral Doses of Dichloroacetate Results in Upregulation of Hepatic Glutathione Transferases and NAD(P)H Dehydrogenase [Quinone] 1.Drug Metab Dispos. 2020 Nov;48(11):1224-1230. doi: 10.1124/dmd.120.000143. Epub 2020 Sep 1. Drug Metab Dispos. 2020. PMID: 32873592 Free PMC article.
-
Phenylalanine-induced leucopenia in genetic and dichloroacetic acid generated deficiency of glutathione transferase Zeta.Biochem Pharmacol. 2009 Apr 15;77(8):1358-63. doi: 10.1016/j.bcp.2009.01.017. Epub 2009 Feb 2. Biochem Pharmacol. 2009. PMID: 19426674
-
Deficiency of glutathione transferase zeta causes oxidative stress and activation of antioxidant response pathways.Mol Pharmacol. 2006 Feb;69(2):650-7. doi: 10.1124/mol.105.018911. Epub 2005 Nov 8. Mol Pharmacol. 2006. PMID: 16278372
-
Human glutathione transferase zeta.Methods Enzymol. 2005;401:61-77. doi: 10.1016/S0076-6879(05)01004-9. Methods Enzymol. 2005. PMID: 16399379 Review.
-
Glutathione transferase zeta: discovery, polymorphic variants, catalysis, inactivation, and properties of Gstz1-/- mice.Drug Metab Rev. 2011 May;43(2):215-25. doi: 10.3109/03602532.2010.549132. Epub 2011 Feb 8. Drug Metab Rev. 2011. PMID: 21303221 Review.
Cited by
-
Proteomic analysis of glutathione S-transferase isoforms in mouse liver mitochondria.World J Gastroenterol. 2012 Jul 14;18(26):3435-42. doi: 10.3748/wjg.v18.i26.3435. World J Gastroenterol. 2012. PMID: 22807614 Free PMC article.
-
GSTO1-1 plays a pro-inflammatory role in models of inflammation, colitis and obesity.Sci Rep. 2017 Dec 19;7(1):17832. doi: 10.1038/s41598-017-17861-6. Sci Rep. 2017. PMID: 29259211 Free PMC article.
-
The Genetic Architecture of Murine Glutathione Transferases.PLoS One. 2016 Feb 1;11(2):e0148230. doi: 10.1371/journal.pone.0148230. eCollection 2016. PLoS One. 2016. PMID: 26829228 Free PMC article.
-
RNA-sequencing quantification of hepatic ontogeny and tissue distribution of mRNAs of phase II enzymes in mice.Drug Metab Dispos. 2013 Apr;41(4):844-57. doi: 10.1124/dmd.112.050211. Epub 2013 Feb 4. Drug Metab Dispos. 2013. PMID: 23382457 Free PMC article.
-
Whole exome sequencing in the Framingham Heart Study identifies rare variation in HYAL2 that influences platelet aggregation.Thromb Haemost. 2017 Jun 2;117(6):1083-1092. doi: 10.1160/TH16-09-0677. Epub 2017 Mar 16. Thromb Haemost. 2017. PMID: 28300864 Free PMC article.
References
-
- Mannervik B, Danielson UH. Glutathione transferases—structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337. - PubMed
-
- Blackburn AC, Woollatt E, Sutherland GR, Board PG. Characterization and chromosome location of the gene GSTZ1 encoding the human zeta class glutathione transferase and maleylacetoacetate isomerase. Cytogenet Cell Genet. 1998;83:109–114. - PubMed
-
- Fernandez-Canon JM, Penalva MA. Characterization of a fungal maleylacetoacetate isomerase gene and identification of its human homologue. J Biol Chem. 1998;273:329–337. - PubMed
-
- Johansson AS, Mannervik B. Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J Biol Chem. 2001;276:33061–33065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

