Muscle satellite cells adopt divergent fates: a mechanism for self-renewal?
- PMID: 15277541
- PMCID: PMC2172269
- DOI: 10.1083/jcb.200312007
Muscle satellite cells adopt divergent fates: a mechanism for self-renewal?
Abstract
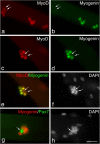
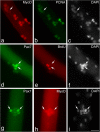
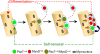
Growth, repair, and regeneration of adult skeletal muscle depends on the persistence of satellite cells: muscle stem cells resident beneath the basal lamina that surrounds each myofiber. However, how the satellite cell compartment is maintained is unclear. Here, we use cultured myofibers to model muscle regeneration and show that satellite cells adopt divergent fates. Quiescent satellite cells are synchronously activated to coexpress the transcription factors Pax7 and MyoD. Most then proliferate, down-regulate Pax7, and differentiate. In contrast, other proliferating cells maintain Pax7 but lose MyoD and withdraw from immediate differentiation. These cells are typically located in clusters, together with Pax7-ve progeny destined for differentiation. Some of the Pax7+ve/MyoD-ve cells then leave the cell cycle, thus regaining the quiescent satellite cell phenotype. Significantly, noncycling cells contained within a cluster can be stimulated to proliferate again. These observations suggest that satellite cells either differentiate or switch from terminal myogenesis to maintain the satellite cell pool.
Figures



References
-
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. - PubMed
-
- Baroffio, A., M. Hamann, L. Bernheim, M.L. Bochaton-Piallat, G. Gabbiani, and C.R. Bader. 1996. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 60:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

