Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments
- PMID: 15277575
- PMCID: PMC2229626
- DOI: 10.1085/jgp.200409062
Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments
Abstract
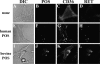
Retinal pigment epithelial (RPE) cells mediate the recognition and clearance of effete photoreceptor outer segments (POS), a process central to the maintenance of normal vision. Given the emerging importance of Toll-like receptors (TLRs) in transmembrane signaling in response to invading pathogens as well as endogenous substances, we hypothesized that TLRs are associated with RPE cell management of POS. TLR4 clusters on human RPE cells in response to human, but not bovine, POS. However, TLR4 clustering could be inhibited by saturating concentrations of an inhibitory anti-TLR4 mAb. Furthermore, human POS binding to human RPE cells elicited transmembrane metabolic and calcium signals within RPE cells, which could be blocked by saturating doses of an inhibitory anti-TLR4 mAb. However, the heterologous combination of bovine POS and human RPE did not trigger these signals. The pattern recognition receptor CD36 collected at the POS-RPE cell interface for both homologous and heterologous samples, but human TLR4 only collected at the human POS-human RPE cell interface. Kinetic experiments of human POS binding to human RPE cells revealed that CD36 arrives at the POS-RPE interface followed by TLR4 accumulation within 2 min. Metabolic and calcium signals immediately follow. Similarly, the production of reactive oxygen metabolites (ROMs) was observed for the homologous human system, but not the heterologous bovine POS-human RPE cell system. As (a) the bovine POS/human RPE combination did not elicit TLR4 accumulation, RPE signaling, or ROM release, (b) TLR4 arrives at the POS-RPE cell interface just before signaling, (c) TLR4 blockade with an inhibitory anti-TLR4 mAb inhibited TLR4 clustering, signaling, and ROM release in the human POS-human RPE system, and (d) TLR4 demonstrates similar clustering and signaling responses to POS in confluent RPE monolayers, we suggest that TLR4 of RPE cells participates in transmembrane signaling events that contribute to the management of human POS.
Figures






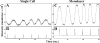
Similar articles
-
Human, but not bovine, photoreceptor outer segments prime human retinal pigment epithelial cells for metabolic activation and massive oxidant release in response to lipopolysaccharide and interferon-gamma.Exp Eye Res. 2004 Sep;79(3):431-5. doi: 10.1016/j.exer.2004.06.005. Exp Eye Res. 2004. PMID: 15336507
-
Formation of lipofuscin in cultured retinal pigment epithelial cells exposed to pre-oxidized photoreceptor outer segments.APMIS. 1996 Apr;104(4):272-9. doi: 10.1111/j.1699-0463.1996.tb00717.x. APMIS. 1996. PMID: 8645466
-
Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments.Invest Ophthalmol Vis Sci. 2003 Apr;44(4):1775-82. doi: 10.1167/iovs.02-0742. Invest Ophthalmol Vis Sci. 2003. PMID: 12657621
-
Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments.Exp Eye Res. 2007 Apr;84(4):635-45. doi: 10.1016/j.exer.2006.11.015. Epub 2006 Dec 31. Exp Eye Res. 2007. PMID: 17292886
-
[Retinal pigment epithelial cell transplantation: perspective].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):982-1006. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022310 Review. Japanese.
Cited by
-
Lipopolysaccharide Promotes Choroidal Neovascularization by Up-Regulation of CXCR4 and CXCR7 Expression in Choroid Endothelial Cell.PLoS One. 2015 Aug 19;10(8):e0136175. doi: 10.1371/journal.pone.0136175. eCollection 2015. PLoS One. 2015. PMID: 26288180 Free PMC article.
-
Caveolins and caveolae in ocular physiology and pathophysiology.Prog Retin Eye Res. 2017 Jan;56:84-106. doi: 10.1016/j.preteyeres.2016.09.005. Epub 2016 Sep 21. Prog Retin Eye Res. 2017. PMID: 27664379 Free PMC article. Review.
-
Hepcidin expression in mouse retina and its regulation via lipopolysaccharide/Toll-like receptor-4 pathway independent of Hfe.Biochem J. 2008 Apr 1;411(1):79-88. doi: 10.1042/BJ20071377. Biochem J. 2008. PMID: 18042040 Free PMC article.
-
Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis.J Infect Dis. 2010 Jan 15;201(2):255-63. doi: 10.1086/649589. J Infect Dis. 2010. PMID: 19995266 Free PMC article.
-
Photoreceptor cells constitutively express functional TLR4.J Neuroimmunol. 2011 Jan;230(1-2):183-7. doi: 10.1016/j.jneuroim.2010.07.022. J Neuroimmunol. 2011. PMID: 20801528 Free PMC article.
References
-
- Akashi, S., H. Ogata, F. Kirikae, T. Kirikae, K. Kawasaki, M. Nishijima, R. Shimazu, Y. Nagai, K. Fukudome, M. Kimoto, and K. Miyake. 2000. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem. Biophys. Res. Commun. 268:172–177. - PubMed
-
- Akira, S. 2000. Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28:551–556. - PubMed
-
- Barton, G.M., and R. Medzhitov. 2002. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 270:81–92. - PubMed
-
- Beg, A.A. 2002. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23:509–512. - PubMed
-
- Bihl, F., L. Lariviere, S.T. Qureshi, L. Flaherty, and D. Malo. 2001. LPS-hyporesponsiveness of mnd mice is associated with a mutation in Toll-like receptor 4. Genes Immun. 2:56–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

