In vivo positron-emission tomography imaging of progression and transformation in a mouse model of mammary neoplasia
- PMID: 15277673
- PMCID: PMC509219
- DOI: 10.1073/pnas.0404396101
In vivo positron-emission tomography imaging of progression and transformation in a mouse model of mammary neoplasia
Abstract
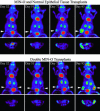
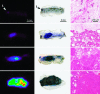
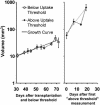
Imaging mouse models of human cancer promises more effective analysis of tumor progression and reduction of the number of animals needed for statistical power in preclinical therapeutic intervention trials. This study utilizes positron emission tomography imaging of 2-[18F]-fluoro-deoxy-D-glucose to monitor longitudinal development of mammary intraepithelial neoplasia outgrowths in immunocompetent FVB/NJ mice. The mammary intraepithelial neoplasia outgrowth tissues mimic the progression of breast cancer from premalignant ductal carcinoma in situ to invasive carcinoma. Progression of disease is clearly evident in the positron emission tomography images, and tracer uptake correlates with histological evaluation. Furthermore, quantitative markers of disease extracted from the images can be used to track proliferation and progression in vivo over multiple time points.
Figures


References
-
- Cardiff, R. D. & Muller, W. J. (1993) Cancer Surv. 16, 97-113. - PubMed
-
- Cardiff, R. D., Moghanaki, D. & Jensen, R. A. (2000) J. Mamm. Gland Biol. Neoplasia 5, 421-437. - PubMed
-
- Kavanaugh, C. & Green, J. E. (2003) J. Nutr. 133, 2404S-2409S. - PubMed
-
- Cardiff, R. D. (2001) Microsc. Res. Tech. 52, 224-230. - PubMed
-
- Cardiff, R. D. (2003) Comp. Med. 53, 250-253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

