Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer
- PMID: 15279716
- PMCID: PMC1872001
- DOI: 10.1215/S1152851703000668
Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer
Abstract
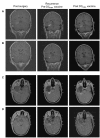
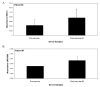
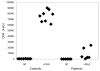
We conducted a phase 1 study of 9 pediatric patients with recurrent brain tumors using monocyte-derived dendritic cells pulsed with tumor RNA to produce antitumor vaccine (DCRNA) preparations. The objectives of this study included (1) establishing safety and feasibility and (2) measuring changes in general, antigen-specific, and tumor-specific immune responses after DCRNA. Dendritic cells were derived from freshly isolated monocytes after 7 days of culture with IL-4 and granulocyte-macrophage colony-stimulating factor, pulsed with autologous tumor RNA, and then cryopreserved. Patients received at least 3 vaccines, each consisting of an intravenous and an intradermal administration at biweekly intervals. The study showed that this method for producing and administering DCRNA from a single leukapheresis product was both feasible and safe in this pediatric brain tumor population. Immune function at the time of enrollment into the study was impaired in all patients tested. While humoral responses to recall antigens (diphtheria and tetanus) were intact in all patients, cellular responses to mitogen and recall antigens were below normal. Following DCRNA vaccine, 2 of 7 patients showed stable clinical disease and 1 of 7 showed a partial response. Two of 7 patients who were tested showed a tumor-specific immune response to DCRNA. This study showed that DCRNA vaccines are both safe and feasible in children with tumors of the central nervous system with a single leukapheresis.
Figures

References
-
- Ashley DM, Bigner DD. Recent advances in the biology of central nervous system tumors. Curr Opin Neurol. 1997;10:445–451. - PubMed
-
- Cinque S, Willems J, Depraetere S, Vermeire L, Joniau M. “In vitro” effect of interleukin-1 beta on human glioma cell lines: Regulation of cell proliferation and IL-6 production”. Immunol Lett. 1992;34:267–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

