K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels
- PMID: 15280082
- PMCID: PMC1382024
- DOI: 10.1080/10739680490425985
K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels
Abstract
Objective: The mechanism by which elevated extracellular potassium ion concentration ([K+]o) causes dilation of skeletal muscle arterioles was evaluated.
Methods: Arterioles (n = 111) were hand-dissected from hamster cremaster muscles, cannulated with glass micropipettes and pressurized to 80 cm H2O for in vitro study. The vessels were superfused with physiological salt solution containing 5 mM KCl, which could be rapidly switched to test solutions containing elevated [K+]o and/or inhibitors. The authors measured arteriolar diameter with a computer-based diameter tracking system, vascular smooth muscle cell membrane potential with sharp micropipettes filled with 200 mM KCl, and changes in intracellular Ca2+ concentration ([Ca2+]i) with Fura 2. Membrane currents and potentials also were measured in enzymatically isolated arteriolar muscle cells using patch clamp techniques. The role played by inward rectifier K+ (KIR) channels was tested using Ba2+ as an inhibitor. Ouabain and substitution of extracellular Na+ with Li+ were used to examine the function of the Na+/K+ ATPase.
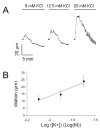
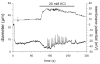
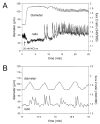
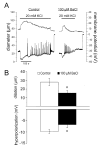
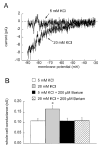
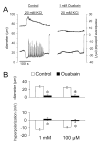
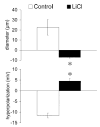
Results: Elevation of [K+]o from 5 mM up to 20 mM caused transient dilation of isolated arterioles (27 +/- 1 microm peak dilation when [K+]o was elevated from 5 to 20 mM, n = 105, p <.05). This dilation was preceded by transient membrane hyperpolarization (10 +/-1 mV, n = 23, p <.05) and by a fall in [Ca2+]i as indexed by a decrease in the Fura 2 fluorescence ratio of 22 +/- 5% (n = 4, p <.05). Ba(2+) (50 or 100 microM) attenuated the peak dilation (40 +/- 8% inhibition, n = 22) and hyperpolarization (31 +/- 12% inhibition, n = 7, p <.05) and decreased the duration of responses by 37 +/-11% (n = 20, p < 0.05). Both ouabain (1 mM or 100 microM) and replacement of Na+ with Li+ essentially abolished both the hyperpolarization and vasodilation.
Conclusions: Elevated [K+]o causes transient vasodilation of skeletal muscle arterioles that appears to be an intrinsic property of the arterioles. The results suggest that K+-induced dilation involves activation of both the Na+/K+ ATPase and KIR channels, leading to membrane hyperpolarization, a fall in [Ca2+]i, and culminating in vasodilation. The Na+/K+ ATPase appears to play the major role and is largely responsible for the transient nature of the response to elevated [K+]o, whereas KIR channels primarily affect the duration and kinetics of the response.
Figures


References
-
- Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res. 2000;37:568–575. - PubMed
-
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275(5 Pt 2):F633–F650. - PubMed
-
- Duling BR. Effects of potassium ion on the microcirculation of the hamster. Circ Res. 1975;37:325–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

