Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy
- PMID: 15280421
- PMCID: PMC2211983
- DOI: 10.1084/jem.20040165
Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy
Abstract
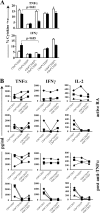
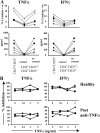
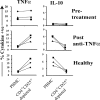
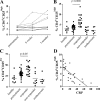
Regulatory T cells have been clearly implicated in the control of disease in murine models of autoimmunity. The paucity of data regarding the role of these lymphocytes in human autoimmune disease has prompted us to examine their function in patients with rheumatoid arthritis (RA). Regulatory (CD4(+)CD25(+)) T cells isolated from patients with active RA displayed an anergic phenotype upon stimulation with anti-CD3 and anti-CD28 antibodies, and suppressed the proliferation of effector T cells in vitro. However, they were unable to suppress proinflammatory cytokine secretion from activated T cells and monocytes, or to convey a suppressive phenotype to effector CD4(+)CD25(-) T cells. Treatment with antitumor necrosis factor alpha (TNFalpha; Infliximab) restored the capacity of regulatory T cells to inhibit cytokine production and to convey a suppressive phenotype to "conventional" T cells. Furthermore, anti-TNFalpha treatment led to a significant rise in the number of peripheral blood regulatory T cells in RA patients responding to this treatment, which correlated with a reduction in C reactive protein. These data are the first to demonstrate that regulatory T cells are functionally compromised in RA, and indicate that modulation of regulatory T cells by anti-TNFalpha therapy may be a further mechanism by which this disease is ameliorated.
Figures



Comment in
-
Suppressor T cells in human diseases.J Exp Med. 2004 Aug 2;200(3):273-6. doi: 10.1084/jem.20040812. Epub 2004 Jul 26. J Exp Med. 2004. PMID: 15280423 Free PMC article.
References
-
- Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. - PubMed
-
- Bach, J.F., and J. Francois Bach. 2003. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 3:189–198. - PubMed
-
- Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. - PubMed
-
- Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

