Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs
- PMID: 15280467
- PMCID: PMC479066
- DOI: 10.1128/JVI.78.16.8582-8592.2004
Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs
Abstract
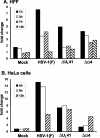
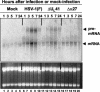
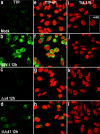
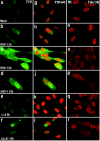
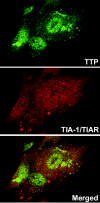
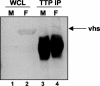
Herpes simplex virus 1 causes a shutoff of cellular protein synthesis through the degradation of RNA that is mediated by the virion host shutoff (Vhs) protein encoded by the U(L)41 gene. We reported elsewhere that the Vhs-dependent degradation of RNA is selective, and we identified RNAs containing AU-rich elements (AREs) that were upregulated after infection but degraded by deadenylation and progressive 3'-to-5' degradation. We also identified upregulated RNAs that were not subject to Vhs-dependent degradation (A. Esclatine, B. Taddeo, L. Evans, and B. Roizman, Proc. Natl. Acad. Sci. USA 101:3603-3608, 2004). Among the latter was the RNA encoding tristetraprolin, a protein that binds AREs and is known to be associated with the degradation of RNAs containing AREs. Prompted by this observation, we examined the status of the ARE binding proteins tristetraprolin and TIA-1/TIAR in infected cells. We report that tristetraprolin was made and accumulated in the cytoplasm of wild-type virus-infected human foreskin fibroblasts as early as 2 h and in HEp-2 cells as early as 6 h after infection. The amounts of tristetraprolin that accumulated in the cytoplasm of cells infected with a mutant virus lacking U(L)41 were significantly lower than those in wild-type virus-infected cells. The localization of tristetraprolin was not modified in cells infected with a mutant lacking the gene encoding infected cell protein 4 (ICP4). TIA-1 and TIAR are two other proteins that are associated with the regulation of ARE-containing RNAs and that normally reside in nuclei. In infected cells, they started to accumulate in the cytoplasm after 6 h of infection. In cells infected with the mutant virus lacking U(L)41, TIA-1/TIAR accumulated in the cytoplasm in granular structures reminiscent of stress granules in a significant percentage of the cells. In addition, an antibody to tristetraprolin coprecipitated the Vhs protein from lysates of cells late in infection. The results indicate that the Vhs-dependent degradation of ARE-containing RNAs correlates with the transactivation, cytoplasmic accumulation, and persistence of tristetraprolin in infected cells.
Figures

Similar articles
-
Tristetraprolin Recruits the Herpes Simplex Virion Host Shutoff RNase to AU-Rich Elements in Stress Response mRNAs To Enable Their Cleavage.J Virol. 2015 May;89(10):5643-50. doi: 10.1128/JVI.00091-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762736 Free PMC article.
-
The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3603-8. doi: 10.1073/pnas.0400354101. Epub 2004 Mar 1. Proc Natl Acad Sci U S A. 2004. PMID: 14993598 Free PMC article.
-
Post-transcriptional processing of cellular RNAs in herpes simplex virus-infected cells.Biochem Soc Trans. 2004 Nov;32(Pt 5):697-701. doi: 10.1042/BST0320697. Biochem Soc Trans. 2004. PMID: 15493991 Review.
-
The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3' degradation of its RNA, preclude expression of the gene.J Virol. 2003 Jun;77(11):6178-87. doi: 10.1128/jvi.77.11.6178-6187.2003. J Virol. 2003. PMID: 12743274 Free PMC article.
-
AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors.Biochem Soc Trans. 2002 Nov;30(Pt 6):952-8. doi: 10.1042/bst0300952. Biochem Soc Trans. 2002. PMID: 12440953 Review.
Cited by
-
mRNA decay during herpes simplex virus (HSV) infections: mutations that affect translation of an mRNA influence the sites at which it is cleaved by the HSV virion host shutoff (Vhs) protein.J Virol. 2013 Jan;87(1):94-109. doi: 10.1128/JVI.01557-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077305 Free PMC article.
-
Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation.PLoS Pathog. 2015 Feb 6;11(2):e1004659. doi: 10.1371/journal.ppat.1004659. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25658430 Free PMC article.
-
Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions.J Virol. 2007 Feb;81(3):1148-61. doi: 10.1128/JVI.01812-06. Epub 2006 Nov 8. J Virol. 2007. PMID: 17093196 Free PMC article.
-
Posttranscriptional suppression of interleukin-6 production by human cytomegalovirus.J Virol. 2005 Jan;79(1):472-85. doi: 10.1128/JVI.79.1.472-485.2005. J Virol. 2005. PMID: 15596840 Free PMC article.
-
Role of KSRP in control of type I interferon and cytokine expression.J Interferon Cytokine Res. 2014 Apr;34(4):267-74. doi: 10.1089/jir.2013.0143. J Interferon Cytokine Res. 2014. PMID: 24697204 Free PMC article. Review.
References
-
- Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. - PubMed
-
- Arlt, A., O. Grobe, A. Sieke, M. L. Kruse, U. R. Folsch, W. E. Schmidt, and H. Schafer. 2001. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in HeLa cells. Oncogene 20:69-76. - PubMed
-
- Bevilacqua, A., M. C. Ceriani, S. Capaccioli, and A. Nicolin. 2003. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J. Cell Physiol. 195:356-372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

