Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV
- PMID: 15280483
- PMCID: PMC479099
- DOI: 10.1128/JVI.78.16.8753-8760.2004
Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV
Abstract
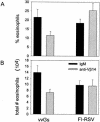
Mice immunized with respiratory syncytial virus (RSV) G glycoprotein or with formalin-inactivated RSV (FI-RSV) exhibit severe disease following RSV challenge. This results in type 2 cytokine production and pulmonary eosinophilia, both hallmarks of vaccine-enhanced disease. RSV G-induced T-cell responses were shown to be restricted to CD4(+) T cells expressing Vbeta14 in the T-cell receptor (TCR), and the deletion of these T cells resulted in less severe disease. We therefore examined the role of Vbeta14(+) T cells in FI-RSV-induced disease. BALB/c mice were immunized with vaccinia virus expressing secreted RSV G (vvGs) or with FI-RSV. At the time of challenge with live RSV, mice were injected with antibody to the Vbeta14 component of the TCR. vvGs-immunized mice treated with anti-Vbeta14 had reduced cytokine levels in the lung. Eosinophil recruitment to the lung was also significantly reduced. In contrast, depletion of Vbeta14(+) T cells in FI-RSV-immunized mice had little impact on cytokine production or pulmonary eosinophilia. An analysis of TCR Vbeta chain usage confirmed a bias toward Vbeta14 expression on CD4(+) T cells from vvGs-immunized mice, whereas the CD4(+) T cells in FI-RSV-immunized mice expressed a diverse array of Vbeta chains. These data show that although FI-RSV and vvGs induce responses resulting in similar immunopathology, the T-cell repertoire mediating the response is different for each immunogen and suggest that the immune responses elicited by RSV G are not the basis for FI-RSV vaccine-enhanced disease.
Figures



Similar articles
-
IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge.J Immunol. 2003 Feb 15;170(4):2037-45. doi: 10.4049/jimmunol.170.4.2037. J Immunol. 2003. PMID: 12574374
-
Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV.J Virol. 2004 Jun;78(11):6024-32. doi: 10.1128/JVI.78.11.6024-6032.2004. J Virol. 2004. PMID: 15141000 Free PMC article.
-
Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells.J Virol. 2015 Nov;89(22):11692-705. doi: 10.1128/JVI.02018-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355098 Free PMC article.
-
Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection.Pediatr Infect Dis J. 2004 Jan;23(1 Suppl):S46-57. doi: 10.1097/01.inf.0000108192.94692.d2. Pediatr Infect Dis J. 2004. PMID: 14730270 Review.
-
Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease.Immunol Res. 2007;39(1-3):225-39. doi: 10.1007/s12026-007-0071-6. Immunol Res. 2007. PMID: 17917067 Review.
Cited by
-
Host Components Contributing to Respiratory Syncytial Virus Pathogenesis.Front Immunol. 2019 Sep 12;10:2152. doi: 10.3389/fimmu.2019.02152. eCollection 2019. Front Immunol. 2019. PMID: 31572372 Free PMC article. Review.
-
Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease.Clin Vaccine Immunol. 2015 Dec 16;23(3):189-95. doi: 10.1128/CVI.00609-15. Clin Vaccine Immunol. 2015. PMID: 26677198 Free PMC article. Review.
-
Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus.Expert Rev Vaccines. 2008 Oct;7(8):1239-55. doi: 10.1586/14760584.7.8.1239. Expert Rev Vaccines. 2008. PMID: 18844597 Free PMC article. Review.
-
Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants.PLoS Med. 2006 Dec;3(12):e525. doi: 10.1371/journal.pmed.0030525. PLoS Med. 2006. PMID: 17194199 Free PMC article.
-
Immunogenicity and efficacy of alphavirus-derived replicon vaccines for respiratory syncytial virus and human metapneumovirus in nonhuman primates.Vaccine. 2016 Feb 10;34(7):950-6. doi: 10.1016/j.vaccine.2015.12.045. Epub 2016 Jan 7. Vaccine. 2016. PMID: 26772634 Free PMC article.
References
-
- Bangham, C. R. M., P. J. M. Openshaw, A. Ball, A. M. Q. King, G. W. Wertz, and B. A. Askonas. 1986. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotien (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J. Immunol. 137:3973-3977. - PubMed
-
- Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473-2480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

