Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex
- PMID: 15280493
- PMCID: PMC479101
- DOI: 10.1128/JVI.78.16.8852-8859.2004
Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex
Abstract
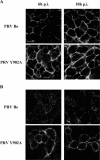
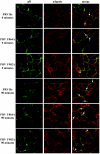
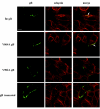
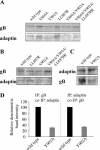
The cytoplasmic domain of pseudorabies virus (PRV) glycoprotein B (gB) contains three putative internalization motifs. Previously, we demonstrated that the tyrosine-based YQRL motif at positions 902 to 905, but not the YMSI motif at positions 864 to 867 or the LL doublet at positions 887 and 888, is required for correct functioning of gB during antibody-mediated internalization of PRV cell surface-bound glycoproteins. In the present study, we demonstrate that the YQRL motif is also crucial to allow spontaneous internalization of PRV gB, and thus, that spontaneous and antibody-mediated internalizations of PRV gB occur through closely related mechanisms. Furthermore, we found that PRV gB colocalizes with the cellular clathrin-associated AP-2 adaptor complex and that this colocalization depends on the YQRL motif. In addition, by coimmunoprecipitation assays, we found that during both spontaneous and antibody-dependent internalization, PRV gB physically interacts with AP-2, and that efficient interaction between gB and AP-2 required an intact YQRL motif. Collectively, these findings demonstrate for the first time that during internalization of an alphaherpesvirus envelope protein, i.e., PRV gB, a specific amino acid sequence in the cytoplasmic tail of the protein interacts with AP-2 and may constitute a common AP-2-mediated mechanism of internalization of alphaherpesvirus envelope proteins.
Figures
Similar articles
-
A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread.J Virol. 2002 Jul;76(13):6845-51. doi: 10.1128/jvi.76.13.6845-6851.2002. J Virol. 2002. PMID: 12050399 Free PMC article.
-
Pseudorabies virus glycoprotein gD contains a functional endocytosis motif that acts in concert with an endocytosis motif in gB to drive internalization of antibody-antigen complexes from the surface of infected monocytes.J Virol. 2005 Jun;79(11):7248-54. doi: 10.1128/JVI.79.11.7248-7254.2005. J Virol. 2005. PMID: 15890963 Free PMC article.
-
Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity.J Virol. 2000 Aug;74(15):7137-45. doi: 10.1128/jvi.74.15.7137-7145.2000. J Virol. 2000. PMID: 10888654 Free PMC article.
-
Functional Relevance of the N-Terminal Domain of Pseudorabies Virus Envelope Glycoprotein H and Its Interaction with Glycoprotein L.J Virol. 2017 Apr 13;91(9):e00061-17. doi: 10.1128/JVI.00061-17. Print 2017 May 1. J Virol. 2017. PMID: 28228592 Free PMC article.
-
The role of virion membrane protein endocytosis in the herpesvirus life cycle.J Clin Virol. 2000 Aug;17(2):69-82. doi: 10.1016/s1386-6532(00)00084-6. J Clin Virol. 2000. PMID: 10942087 Review.
Cited by
-
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses.Viruses. 2015 Sep 18;7(9):5084-114. doi: 10.3390/v7092861. Viruses. 2015. PMID: 26393641 Free PMC article. Review.
-
Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus.J Virol. 2009 Aug;83(16):7796-804. doi: 10.1128/JVI.00745-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19494011 Free PMC article.
-
Replication of feline coronaviruses in peripheral blood monocytes.Arch Virol. 2005 Dec;150(12):2483-500. doi: 10.1007/s00705-005-0598-6. Epub 2005 Aug 1. Arch Virol. 2005. PMID: 16052283 Free PMC article.
-
TMEM41B Is an Interferon-Stimulated Gene That Promotes Pseudorabies Virus Replication.J Virol. 2023 Jun 29;97(6):e0041223. doi: 10.1128/jvi.00412-23. Epub 2023 May 31. J Virol. 2023. PMID: 37255475 Free PMC article.
-
Viral Interactions with Adaptor-Protein Complexes: A Ubiquitous Trait among Viral Species.Int J Mol Sci. 2021 May 17;22(10):5274. doi: 10.3390/ijms22105274. Int J Mol Sci. 2021. PMID: 34067854 Free PMC article. Review.
References
-
- Ben-Porat, T., J. M. Demarchi, B. Lomnicizi, and A. S. Kaplan. 1986. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology 154:325-334. - PubMed
-
- Berthomme, H., B. Jacquemont, and A. Epstein. 1993. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 193:1028-1032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

