Vaccinia virus morphogenesis: a13 phosphoprotein is required for assembly of mature virions
- PMID: 15280497
- PMCID: PMC479082
- DOI: 10.1128/JVI.78.16.8885-8901.2004
Vaccinia virus morphogenesis: a13 phosphoprotein is required for assembly of mature virions
Abstract
The 70-amino-acid A13L protein is a component of the vaccinia virus membrane. We demonstrate here that the protein is expressed at late times of infection, undergoes phosphorylation at a serine residue(s), and becomes encapsidated in a monomeric form. Phosphorylation is dependent on Ser40, which lies within the proline-rich motif SPPP. Because phosphorylation of the A13 protein is only minimally affected by disruption of the viral F10 kinase or H1 phosphatase, a cellular kinase is likely to be involved. We generated an inducible recombinant in which A13 protein expression is dependent upon the inclusion of tetracycline in the culture medium. Repression of the A13L protein spares the biochemical progression of the viral life cycle but arrests virion morphogenesis. Virion assembly progresses through the formation of immature virions (IVs); however, these virions do not acquire nucleoids, and DNA crystalloids accumulate in the cytoplasm. Further development into intracellular mature virions is blocked, causing a 1,000-fold decrease in the infectious virus yield relative to that obtained in the presence of the inducer. We also determined that the temperature-sensitive phenotype of the viral mutant Cts40 is due to a nucleotide transition within the A13L gene that causes a Thr(48)-->Ile substitution. This substitution disrupts the function of the A13 protein but does not cause thermolability of the protein; at the nonpermissive temperature, virion morphogenesis arrests at the stage of IV formation. The A13L protein, therefore, is part of a newly recognized group of membrane proteins that are dispensable for the early biogenesis of the virion membrane but are essential for virion maturation.
Figures



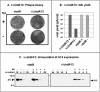

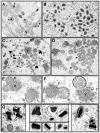

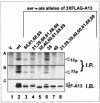

Similar articles
-
Elucidating the essential role of the A14 phosphoprotein in vaccinia virus morphogenesis: construction and characterization of a tetracycline-inducible recombinant.J Virol. 2000 Apr;74(8):3682-95. doi: 10.1128/jvi.74.8.3682-3695.2000. J Virol. 2000. PMID: 10729144 Free PMC article.
-
Vaccinia virus mutants with alanine substitutions in the conserved G5R gene fail to initiate morphogenesis at the nonpermissive temperature.J Virol. 2004 Oct;78(19):10238-48. doi: 10.1128/JVI.78.19.10238-10248.2004. J Virol. 2004. PMID: 15367589 Free PMC article.
-
Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo.J Virol. 2000 Apr;74(7):3353-65. doi: 10.1128/jvi.74.7.3353-3365.2000. J Virol. 2000. PMID: 10708453 Free PMC article.
-
In a nutshell: structure and assembly of the vaccinia virion.Adv Virus Res. 2006;66:31-124. doi: 10.1016/S0065-3527(06)66002-8. Adv Virus Res. 2006. PMID: 16877059 Review.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
Cited by
-
Human antibody responses to the polyclonal Dryvax vaccine for smallpox prevention can be distinguished from responses to the monoclonal replacement vaccine ACAM2000.Clin Vaccine Immunol. 2014 Jun;21(6):877-85. doi: 10.1128/CVI.00035-14. Epub 2014 Apr 23. Clin Vaccine Immunol. 2014. PMID: 24759651 Free PMC article.
-
Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles.J Virol. 2006 Mar;80(5):2127-40. doi: 10.1128/JVI.80.5.2127-2140.2006. J Virol. 2006. PMID: 16474121 Free PMC article.
-
Temporal expression classes and functions of vaccinia virus and mpox (monkeypox) virus genes.mBio. 2025 Apr 9;16(4):e0380924. doi: 10.1128/mbio.03809-24. Epub 2025 Mar 20. mBio. 2025. PMID: 40111027 Free PMC article. Review.
-
Vaccinia H5 is a multifunctional protein involved in viral DNA replication, postreplicative gene transcription, and virion morphogenesis.Virology. 2010 May 25;401(1):49-60. doi: 10.1016/j.virol.2010.01.020. Epub 2010 Mar 5. Virology. 2010. PMID: 20206959 Free PMC article.
-
Viral miniproteins.Annu Rev Microbiol. 2014;68:21-43. doi: 10.1146/annurev-micro-091313-103727. Epub 2014 Apr 10. Annu Rev Microbiol. 2014. PMID: 24742054 Free PMC article. Review.
References
-
- Betakova, T., E. J. Wolffe, and B. Moss. 1999. Membrane topology of the vaccinia virus A17L envelope protein. Virology 261:347-356. - PubMed
-
- Boyle, W. J., G. P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. - PubMed
-
- Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

