The gene encoding the nucleocapsid protein of Gill-associated nidovirus of Penaeus monodon prawns is located upstream of the glycoprotein gene
- PMID: 15280504
- PMCID: PMC479087
- DOI: 10.1128/JVI.78.16.8935-8941.2004
The gene encoding the nucleocapsid protein of Gill-associated nidovirus of Penaeus monodon prawns is located upstream of the glycoprotein gene
Abstract
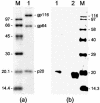
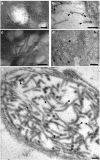
The ORF2 gene of Gill-associated virus (GAV) of Penaeus monodon prawns resides 93 nucleotides downstream of the ORF1a-ORF1b gene and encodes a 144-amino-acid hydrophilic polypeptide (15,998 Da; pI, 9.75) containing 20 basic (14%) and 13 acidic (9%) residues and 19 prolines (13%). Antiserum to a synthetic ORF2 peptide or an Escherichia coli-expressed glutathione S-transferase-ORF2 fusion protein detected a 20-kDa protein in infected lymphoid organ and gill tissues in Western blots. The GAV ORF2 fusion protein antiserum also cross-reacted with the p20 nucleoprotein in virions of the closely related Yellow head virus. By immuno-gold electron microscopy, it was observed that the ORF2 peptide antibody localized to tubular GAV nucleocapsids, often at the ends or at lateral cross sections. As GAV appears to contain only two structural protein genes (ORF2 and ORF3), these data indicate that GAV differs from vertebrate nidoviruses in that the gene encoding the nucleocapsid protein is located upstream of the gene encoding the virion glycoproteins.
Figures


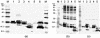
References
-
- Boonyaratpalin, S., K. Supamattaya, J. Kasornchandra, S. Direkbusaracom, U. Aekpanithanpong, and C. Chantanachookin. 1993. Non-occluded baculo-like virus, the causative agent of yellow-head disease in the black tiger shrimp (Penaeus monodon). Fish Pathol. 28:103-109.
-
- Chantanachookin, C., S. Boonyaratpalin, J. Kasornchandra, D. Sataporn, U. Ekpanithanpong, K. Supamataya, S. Riurairatana, and T. W. Flegel. 1993. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis. Aquat. Org. 17:145-157.
-
- Chen, Z., L. Kuo, R. R. Rowland, C. Even, K. S. Faaberg, and P. G. Plagemann. 1993. Sequences of 3′ end of genome and of 5′ end of open reading frame 1a of lactate dehydrogenase-elevating virus and common junction motifs between 5′ leader and bodies of seven subgenomic mRNAs. J. Gen. Virol. 74:643-659. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

