Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex
- PMID: 15280535
- PMCID: PMC511061
- DOI: 10.1073/pnas.0404474101
Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex
Abstract
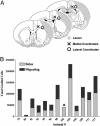
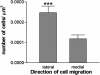
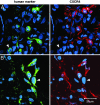
We characterize the survival, migration, and differentiation of human neurospheres derived from CNS stem cells transplanted into the ischemic cortex of rats 7 days after distal middle cerebral artery occlusion. Transplanted neurospheres survived robustly in naive and ischemic brains 4 wk posttransplant. Survival was influenced by proximity of the graft to the stroke lesion and was negatively correlated with the number of IB4-positive inflammatory cells. Targeted migration of the human cells was seen in ischemic animals, with many human cells migrating long distances ( approximately 1.2 mm) predominantly toward the lesion; in naive rats, cells migrated radially from the injection site in smaller number and over shorter distances (0.2 mm). The majority of migrating cells in ischemic rats had a neuronal phenotype. Migrating cells between the graft and the lesion expressed the neuroblast marker doublecortin, whereas human cells at the lesion border expressed the immature neuronal marker beta-tubulin, although a small percentage of cells at the lesion border also expressed glial fibrillary acid protein (GFAP). Thus, transplanted human CNS (hCNS)-derived neurospheres survived robustly in naive and ischemic brains, and the microenvironment influenced their migration and fate.
Figures
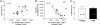


References
-
- American Heart Association (2002) Heart Disease and Stroke Statistics 2003 Update (American Heart Association, Dallas, TX).
-
- Nishino, H. & Borlongan, C. V. (2000) Prog. Brain Res 127, 461-476. - PubMed
-
- Mattsson, B., Sorensen, J. C., Zimmer, J. & Johansson, B. B. (1997) Stroke 28, 1225-1231; discussion 1231-1232. - PubMed
-
- Riolobos, A. S., Heredia, M., de la Fuente, J. A., Criado, J. M., Yajeya, J., Campos, J. & Santacana, M. (2001) Neurobiol. Learn. Mem. 75, 274-292. - PubMed
-
- Savitz, S. I., Rosenbaum, D. M., Dinsmore, J. H., Weschler, L. R. & Caplan, L. R. (2002) Ann. Neurol. 53, 266-275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

