Pauling and Corey's alpha-pleated sheet structure may define the prefibrillar amyloidogenic intermediate in amyloid disease
- PMID: 15280548
- PMCID: PMC511030
- DOI: 10.1073/pnas.0401781101
Pauling and Corey's alpha-pleated sheet structure may define the prefibrillar amyloidogenic intermediate in amyloid disease
Abstract
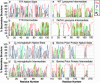
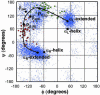
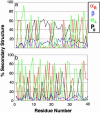
Transthyretin, beta(2)-microglobulin, lysozyme, and the prion protein are four of the best-characterized proteins implicated in amyloid disease. Upon partial acid denaturation, these proteins undergo conformational change into an amyloidogenic intermediate that can self-assemble into amyloid fibrils. Many experiments have shown that pH-mediated changes in structure are required for the formation of the amyloidogeneic intermediate, but it has proved impossible to characterize these conformational changes at high resolution using experimental means. To probe these conformational changes at atomic resolution, we have performed molecular dynamics simulations of these proteins at neutral and low pH. In low-pH simulations of all four proteins, we observe the formation of alpha-pleated sheet secondary structure, which was first proposed by L. Pauling and R. B. Corey [(1951) Proc. Natl. Acad. Sci. USA 37, 251-256]. In all beta-sheet proteins, transthyretin and beta(2)-microglobulin, alpha-pleated sheet structure formed over the strands that are highly protected in hydrogen-exchange experiments probing amyloidogenic conditions. In lysozyme and the prion protein, alpha-sheets formed in the specific regions of the protein implicated in the amyloidogenic conversion. We propose that the formation of alpha-pleated sheet structure may be a common conformational transition in amyloidosis.
Figures

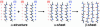
References
-
- Carrell, R. W. & Gooptu, B. (1998) Curr. Opin. Struct. Biol. 6, 799–909. - PubMed
-
- Kelly, J. (1998) Curr. Opin. Struct. Biol. 8, 101–106. - PubMed
-
- Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J. S., Taddei, N, Ramponi, G., Dobson, C. M. & Stefani, M. (2002) Nature 416, 507–511. - PubMed
-
- Chiti, F., Stefani, M., Taddei, N., Ramponi, G. & Dobson, C. M. (2003) Nature 424, 805–808. - PubMed
-
- Saraiva, M. J. M. (2001) Hum. Mutat. 17, 493–503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

