Serum CYFRA 21-1 (cytokeratin-19 fragments) is a useful tumour marker for detecting disease relapse and assessing treatment efficacy in breast cancer
- PMID: 15280913
- PMCID: PMC2409884
- DOI: 10.1038/sj.bjc.6602074
Serum CYFRA 21-1 (cytokeratin-19 fragments) is a useful tumour marker for detecting disease relapse and assessing treatment efficacy in breast cancer
Abstract
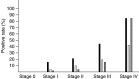
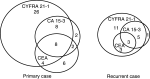
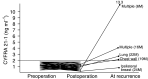
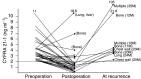
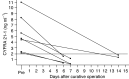
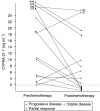
The usefulness of serum CYFRA 21-1 (cytokeratin-19 fragments) in monitoring the recurrence of breast cancer and in evaluating therapeutic effects was studied retrospectively. The sera from 173 patients with primary breast cancer or recurrent disease were measured for CYFRA 21-1, carcinoembryonic antigen (CEA), and carbohydrate antigen 15-3 (CA 15-3) levels. The positive rates of serum CYFRA 21-1 for stage IV (n=12) or recurrent disease (n=26) were 83.3 and 84.6%, respectively, while those of serum CEA were 41.7 and 26.9%, and those of serum CA 15-3 were 83.3 and 34.6%. The elevated preoperative levels of serum CYFRA 21-1 decreased to normal levels after curative operation, whereas the levels remained abnormally high after noncurative operation. There was a significantly high frequency of recurrence in patients with elevated levels of serum CYFRA 21-1 preoperatively compared to those with normal levels of the marker preoperatively. The serum CYFRA 21-1 levels were well correlated with response to chemotherapy. The positive rate of serum CYFRA 21-1 alone was higher than that of an assay combining CEA with CA 15-3, in both primary and recurrent cases (28.8 vs 18.8 and 84.6 vs 46.2%, respectively). These observations suggest that serum CYFRA 21-1 may be a reliable marker of recurrence or therapeutic efficacy.
Figures
Similar articles
-
Serum cytokeratin fragment 21.1 (CYFRA 21.1) as tumour marker for breast cancer: comparison with carbohydrate antigen 15.3 (CA 15.3) and carcinoembryonic antigen (CEA).Clin Chem Lab Med. 2002 Mar;40(3):298-303. doi: 10.1515/CCLM.2002.047. Clin Chem Lab Med. 2002. PMID: 12005221
-
Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer.Cancer. 2006 Dec 15;107(12):2842-9. doi: 10.1002/cncr.22330. Cancer. 2006. PMID: 17103443
-
[The importance of the tumor marker CYFRA 21-1 in patients with lung cancer after surgery or chemotherapy].Hell J Nucl Med. 2007 Jan-Apr;10(1):62-6. Hell J Nucl Med. 2007. PMID: 17450257 Clinical Trial. Greek, Modern.
-
[Tumor markers in breast cancer].Gan To Kagaku Ryoho. 2001 Jul;28(7):1035-40. Gan To Kagaku Ryoho. 2001. PMID: 11478135 Review. Japanese.
-
The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature.Crit Rev Oncol Hematol. 2008 Apr;66(1):10-20. doi: 10.1016/j.critrevonc.2007.09.002. Epub 2007 Oct 26. Crit Rev Oncol Hematol. 2008. PMID: 17964182 Review.
Cited by
-
[Diagnostic Value of CYFRA 21-1 Measurement in Fine-Needle Aspiration Washouts for Detection of Axillary Recurrence in Postoperative Breast Cancer Patients].Taehan Yongsang Uihakhoe Chi. 2020 Jan;81(1):147-156. doi: 10.3348/jksr.2020.81.1.147. Epub 2020 Jan 31. Taehan Yongsang Uihakhoe Chi. 2020. PMID: 36238108 Free PMC article. Korean.
-
Fine-needle aspirate CYFRA 21-1, an innovative new marker for diagnosis of axillary lymph node metastasis in breast cancer patients.Medicine (Baltimore). 2015 May;94(19):e811. doi: 10.1097/MD.0000000000000811. Medicine (Baltimore). 2015. PMID: 25984666 Free PMC article.
-
Serum Levels of Cyfra 21 in Patients with Benign and Malignant Salivary Gland Tumors.Iran J Otorhinolaryngol. 2017 Jul;29(93):203-208. Iran J Otorhinolaryngol. 2017. PMID: 28819618 Free PMC article.
-
Simple diagnosis of cancer by detecting CEA and CYFRA 21-1 in saliva using electronic sensors.Sci Rep. 2022 Sep 12;12(1):15315. doi: 10.1038/s41598-022-19593-8. Sci Rep. 2022. PMID: 36097151 Free PMC article.
-
Molecular Biomarkers in Cholangiocarcinoma: Focus on Bile.Curr Top Med Chem. 2024;24(8):722-736. doi: 10.2174/0115680266290367240130054142. Curr Top Med Chem. 2024. PMID: 38303538 Review.
References
-
- American Society of Clinical Oncology (2001) 2000 Update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guideline of the American Society of Clinical Oncology. J Clin Oncol 19: 1865–1878 - PubMed
-
- Brotheric I, Robson CN, Browell DA, Shenfine J, White MD, Cunliffe WJ, Shenton BK, Egan M, Webb LA, Lunt LG, Young JR, Higgs MJ (1998) Cytokeratin expression in breast cancer: phenotypic changes associated with disease progression. Cytometry 32: 301–308 - PubMed
-
- Giovanella L, Ceriani L, Giardina G, Bardelli D, Tanzi F, Garancini S (2002) Serum cytokeratin fragment 21.1 (CYFRA 21.1) as tumour marker for breast cancer: comparison with carbohydrate antigen 15.3 (CA 15.3) and carcinoembryonic antigen (CEA). Clin Chem Lab Med 40: 298–303 - PubMed
-
- Molina R, Agusti C, Filella X, Jo J, Joseph J, Gimenez N, Ballesta AM (1994) Study of a new tumor marker, CYFRA 21-1, in malignant and nonmalignant diseases. Tumour Biol 15: 318–325 - PubMed
-
- Moll R, Franke WW, Schiller DL (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31: 11–24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

