A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer
- PMID: 15280930
- PMCID: PMC2364780
- DOI: 10.1038/sj.bjc.6602019
A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer
Abstract
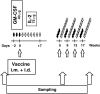
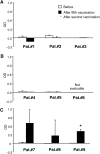
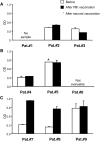
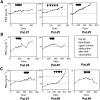
Prostate-specific antigen (PSA) is a serine protease secreted at low levels by normal luminal epithelial cells of the prostate and in significantly higher levels by prostate cancer cells. Therefore, PSA is a potential target for various immunotherapeutical approaches against prostate cancer. DNA vaccination has been investigated as immunotherapy for infectious diseases in patients and for specific treatment of cancer in certain animal models. In animal studies, we have demonstrated that vaccination with plasmid vector pVAX/PSA results in PSA-specific cellular response and protection against tumour challenge. The purpose of the trial was to evaluate the safety, feasibility and biological efficacy of pVAX/PSA vaccine in the clinic. A phase I trial of pVAX/PSA, together with cytokine granulocyte/macrophage-colony stimulating factor (GM-CSF) (Molgramostim) and IL-2 (Aldesleukin) as vaccine adjuvants, was carried out in patients with hormone-refractory prostate cancer. To evaluate the biologically active dose, the vaccine was administered during five cycles in doses of 100, 300 and 900 microg, with three patients in each cohort. Eight patients were evaluable. A PSA-specific cellular immune response, measured by IFN-gamma production against recombinant PSA protein, and a rise in anti-PSA IgG were detected in two of three patients after vaccination in the highest dose cohort. A decrease in the slope of PSA was observed in the two patients exhibiting IFN-gamma production to PSA. No adverse effects (WHO grade >2) were observed in any dose cohort. We demonstrate that DNA vaccination with a PSA-coding plasmid vector, given with GM-CSF and IL-2 to patients with prostate cancer, is safe and in doses of 900 microg the vaccine can induce cellular and humoral immune responses against PSA protein.
Figures
References
-
- Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson AC, Sandstrom E, Wahren B (1998) Cellular cytotoxic response induced by DNA vaccination in HIV-1- infected patients. Lancet 351: 1320–1325 - PubMed
-
- Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL (1991) Measurement of prostate-specific antigen in serum as a screening test for prostate cancer (published erratum appears in N Engl J Med 1991 Oct 31;325(18):1324) (see comments). N Engl J Med 324: 1156–1161 - PubMed
-
- Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY (1997) In vitro generation of human cytotoxic lymphocytes specific for peptides derived from prostate specific antigen. J Natl Cancer Inst 89: 293–300 - PubMed
-
- Coulie PG, van der Bruggen P (2003) T-cell responses of vaccinated cancer patients. Curr Opin Immunol 15: 131–137 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

