Temporal dynamics of ipsilateral and contralateral motor activity during voluntary finger movement
- PMID: 15281139
- PMCID: PMC6872033
- DOI: 10.1002/hbm.20038
Temporal dynamics of ipsilateral and contralateral motor activity during voluntary finger movement
Abstract
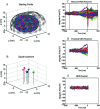
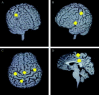
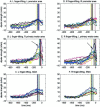
The role of motor activity ipsilateral to movement remains a matter of debate, due in part to discrepancies among studies in the localization of this activity, when observed, and uncertainty about its time course. The present study used magnetoencephalography (MEG) to investigate the spatial localization and temporal dynamics of contralateral and ipsilateral motor activity during the preparation of unilateral finger movements. Eight right-handed normal subjects carried out self-paced finger-lifting movements with either their dominant or nondominant hand during MEG recordings. The Multi-Start Spatial Temporal multi-dipole method was used to analyze MEG responses recorded during the movement preparation and early execution stage (-800 msec to +30 msec) of movement. Three sources were localized consistently, including a source in the contralateral primary motor area (M1) and in the supplementary motor area (SMA). A third source ipsilateral to movement was located significantly anterior, inferior, and lateral to M1, in the premotor area (PMA) (Brodmann area [BA] 6). Peak latency of the SMA and the ipsilateral PMA sources significantly preceded the peak latency of the contralateral M1 source by 60 msec and 52 msec, respectively. Peak dipole strengths of both the SMA and ipsilateral PMA sources were significantly weaker than was the contralateral M1 source, but did not differ from each other. Altogether, the results indicated that the ipsilateral motor activity was associated with premotor function, rather than activity in M1. The time courses of activation in SMA and ipsilateral PMA were consistent with their purported roles in planning movements.
Copyright 2004 Wiley-Liss, Inc.
Figures


Similar articles
-
Finger and face representations in the ipsilateral precentral motor areas in humans.J Neurophysiol. 2005 May;93(5):2950-8. doi: 10.1152/jn.00784.2004. Epub 2004 Dec 29. J Neurophysiol. 2005. PMID: 15625099 Free PMC article.
-
Comparison of unilateral and bilateral complex finger tapping-related activation in premotor and primary motor cortex.Hum Brain Mapp. 2009 Apr;30(4):1397-412. doi: 10.1002/hbm.20610. Hum Brain Mapp. 2009. PMID: 18537112 Free PMC article.
-
Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach.Hum Brain Mapp. 2006 Mar;27(3):213-29. doi: 10.1002/hbm.20178. Hum Brain Mapp. 2006. PMID: 16037985 Free PMC article.
-
Intracerebral ERD/ERS in voluntary movement and in cognitive visuomotor task.Prog Brain Res. 2006;159:311-30. doi: 10.1016/S0079-6123(06)59021-1. Prog Brain Res. 2006. PMID: 17071240
-
Bilateral neuromagnetic activation of human primary sensorimotor cortex in preparation and execution of unilateral voluntary finger movements.Brain Res. 1999 May 8;827(1-2):234-6. doi: 10.1016/s0006-8993(99)01360-8. Brain Res. 1999. PMID: 10320716
Cited by
-
Dipole analysis of magnetoencephalographic data during continuous shape copying.Exp Brain Res. 2006 Apr;170(4):513-21. doi: 10.1007/s00221-005-0234-4. Epub 2005 Nov 23. Exp Brain Res. 2006. PMID: 16328259
-
Cytology and functionally correlated circuits of human posterior cingulate areas.Neuroimage. 2006 Jan 15;29(2):452-66. doi: 10.1016/j.neuroimage.2005.07.048. Epub 2005 Sep 6. Neuroimage. 2006. PMID: 16140550 Free PMC article. Clinical Trial.
-
Effects of intermanual transfer induced by repetitive precision grip on input-output properties of untrained contralateral limb muscles.Exp Brain Res. 2007 Oct;182(4):459-67. doi: 10.1007/s00221-007-1004-2. Epub 2007 Jun 12. Exp Brain Res. 2007. PMID: 17562034
-
Neuronal responses to adverse social threat in healthy human subjects.J Psychiatr Res. 2021 Apr;136:47-53. doi: 10.1016/j.jpsychires.2021.01.015. Epub 2021 Jan 19. J Psychiatr Res. 2021. PMID: 33556904 Free PMC article.
-
Non-invasive ventral cervical magnetoneurography as a proxy of in vivo lipopolysaccharide-induced inflammation.Commun Biol. 2024 Jul 29;7(1):893. doi: 10.1038/s42003-024-06435-8. Commun Biol. 2024. PMID: 39075164 Free PMC article.
References
-
- Aine C, Huang M, Stephen J, Christner R (2000): Multi‐start algorithms for MEG empirical data analysis reliably characterize locations and time‐courses of multiple sources. Neuroimage 12: 159–172. - PubMed
-
- Aizawa H, Mushiake H, Inase M, Tanji J (1990): An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res 82: 219–221. - PubMed
-
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000): Functional MRI cerebral activation and deactivation during finger movement. Neurology 54: 135–142. - PubMed
-
- Ashburner J, Neelin P, Collins DL, Evans AC, Friston KJ (1997): Incorporating prior knowledge into image registration. Neuroimage 6: 344–352. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

