Estimation of multiscale neurophysiologic parameters by electroencephalographic means
- PMID: 15281141
- PMCID: PMC6871818
- DOI: 10.1002/hbm.20032
Estimation of multiscale neurophysiologic parameters by electroencephalographic means
Abstract
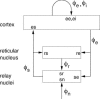
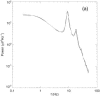
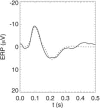
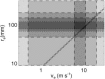
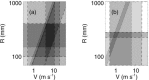
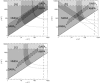
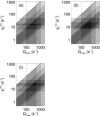
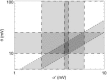
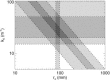
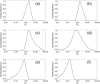
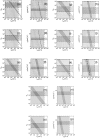
It is shown that new model-based electroencephalographic (EEG) methods can quantify neurophysiologic parameters that underlie EEG generation in ways that are complementary to and consistent with standard physiologic techniques. This is done by isolating parameter ranges that give good matches between model predictions and a variety of experimental EEG-related phenomena simultaneously. Resulting constraints range from the submicrometer synaptic level to length scales of tens of centimeters, and from timescales of around 1 ms to 1 s or more, and are found to be consistent with independent physiologic and anatomic measures. In the process, a new method of obtaining model parameters from the data is developed, including a Monte Carlo implementation for use when not all input data are available. Overall, the approaches used are complementary to other methods, constraining allowable parameter ranges in different ways and leading to much tighter constraints overall. EEG methods often provide the most restrictive individual constraints. This approach opens a new, noninvasive window on quantitative brain analysis, with the ability to monitor temporal changes, and the potential to map spatial variations. Unlike traditional phenomenologic quantitative EEG measures, the methods proposed here are based explicitly on physiology and anatomy.
Copyright 2004 Wiley-Liss, Inc.
Figures
References
-
- Barrionuevo G, Benoit O, Tempier P (1981): Evidence for two types of firing pattern during the sleep‐waking cycle in the reticular thalamic nucleus of the cat. Exp Neurol 72: 486–501. - PubMed
-
- Braitenberg V, Schüz A (1998): Cortex: statistics and geometry of neuronal connectivity (2nd ed). Berlin: Springer.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

