Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits
- PMID: 15282272
- PMCID: PMC6729708
- DOI: 10.1523/JNEUROSCI.1408-04.2004
Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits
Abstract
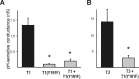
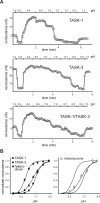
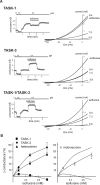
Background potassium currents carried by the KCNK family of two-pore-domain K+ channels are important determinants of resting membrane potential and cellular excitability. TWIK-related acid-sensitive K+ 1 (TASK-1, KCNK3) and TASK-3 (KCNK9) are pH-sensitive subunits of the KCNK family that are closely related and coexpressed in many brain regions. There is accumulating evidence that these two subunits can form heterodimeric channels, but this evidence remains controversial. In addition, a substantial contribution of heterodimeric TASK channels to native currents has not been unequivocally established. In a heterologous expression system, we verified formation of heterodimeric TASK channels and characterized their properties; TASK-1 and TASK-3 were coimmunoprecipitated from membranes of mammalian cells transfected with the channel subunits, and a dominant negative TASK-1(Y191F) construct strongly diminished TASK-3 currents. Tandem-linked heterodimeric TASK channel constructs displayed a pH sensitivity (pK approximately 7.3) in the physiological range closer to that of TASK-1 (pK approximately 7.5) than TASK-3 (pK approximately 6.8). On the other hand, heteromeric TASK channels were like TASK-3 insofar as they were activated by high concentrations of isoflurane (0.8 mm), whereas TASK-1 channels were inhibited. The pH and isoflurane sensitivities of native TASK-like currents in hypoglossal motoneurons, which strongly express TASK-1 and TASK-3 mRNA, were best represented by TASK heterodimeric channels. Moreover, after blocking homomeric TASK-3 channels with ruthenium red, we found a major component of motoneuronal isoflurane-sensitive TASK-like current that could be attributed to heteromeric TASK channels. Together, these data indicate that TASK-1 and TASK-3 subunits coassociate in functional channels, and heteromeric TASK channels provide a substantial component of background K(+) current in motoneurons with distinct modulatory properties.
Figures



References
-
- Bayliss DA, Talley EM, Sirois JE, Lei Q (2001) TASK-1 is a highly modulated pH-sensitive “leak” K+ channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159-174. - PubMed
-
- Bayliss DA, Sirois JE, Talley EM (2003) The TASK family: two-pore domain background K+ channels. Mol Interv 3: 205-219. - PubMed
-
- Chapman CG, Meadows HJ, Godden RJ, Campbell DA, Duckworth M, Kelsell RE, Murdock PR, Randall AD, Rennie GI, Gloger IS (2000) Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res 82: 74-83. - PubMed
-
- Czirjak G, Enyedi P (2002a) Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem 277: 5426-5432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
