Lipid rafts mediate the synaptic localization of alpha-synuclein
- PMID: 15282274
- PMCID: PMC6729723
- DOI: 10.1523/JNEUROSCI.1594-04.2004
Lipid rafts mediate the synaptic localization of alpha-synuclein
Abstract
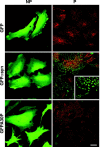
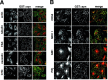
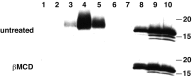
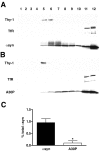
Alpha-synuclein contributes to the pathogenesis of Parkinson's disease (PD), but its precise role in the disorder and its normal function remain poorly understood. Consistent with a presumed role in neurotransmitter release and its prominent deposition in the dystrophic neurites of PD, alpha-synuclein localizes almost exclusively to the nerve terminal. In brain extracts, however, alpha-synuclein behaves as a soluble, monomeric protein. Using a binding assay to characterize the association of alpha-synuclein with cell membranes, we find that alpha-synuclein binds saturably and with high affinity to characteristic intracellular structures that double label for components of lipid rafts. Biochemical analysis demonstrates the interaction of alpha-synuclein with detergent-resistant membranes and reveals a shift in electrophoretic mobility of the raft-associated protein. In addition, the A30P mutation associated with PD disrupts the interaction of alpha-synuclein with lipid rafts. Furthermore, we find that both the A30P mutation and raft disruption redistribute alpha-synuclein away from synapses, indicating an important role for raft association in the normal function of alpha-synuclein and its role in the pathogenesis of PD.
Figures



References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239-252. - PubMed
-
- Breckenridge WC, Morgan IG, Zanetta JP, Vincendon G (1973) Adult rat brain synaptic vesicles. II. Lipid composition. Biochim Biophys Acta 320: 681-686. - PubMed
-
- Brown DA (2001) Lipid droplets: proteins floating on a pool of fat. Curr Biol 11: R446-449. - PubMed
-
- Brown DA, London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14: 111-136. - PubMed
-
- Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533-544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
