Mechanosensory activation of a motor circuit by coactivation of two projection neurons
- PMID: 15282277
- PMCID: PMC6494447
- DOI: 10.1523/JNEUROSCI.1682-04.2004
Mechanosensory activation of a motor circuit by coactivation of two projection neurons
Abstract
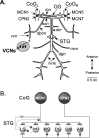
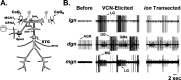
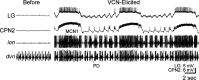
Individual neuronal circuits can generate multiple activity patterns because of the influence of different projection neurons. However, in most systems it has been difficult to identify and assess the relative contribution of all upstream neurons responsible for the activation of any single activity pattern by a behaviorally relevant stimulus. To elucidate this issue, we used the stomatogastric nervous system (STNS) of the crab. The STNS includes the gastric mill (chewing) motor circuit in the stomatogastric ganglion (STG) and no more than 20 projection neurons that innervate the STG. We previously identified at least some (four) of the projection neurons that are activated directly by the ventral cardiac neuron (VCN) system, a population of mechanosensory neurons that activates the gastric mill circuit. Here we show that two of these projection neurons, the previously identified modulatory commissural neuron 1 (MCN1) and commissural projection neuron 2 (CPN2), are necessary and likely sufficient for the initiation/maintenance of the VCN-elicited gastric mill rhythm. Selective inactivation of either MCN1 or CPN2 still enabled a VCN-elicited gastric mill rhythm. However, because MCN1 and CPN2 have different actions on gastric mill neurons, these manipulations resulted in rhythms distinct from each other and from that occurring in the intact system. After removal of both MCN1 and CPN2, VCN stimulation failed to activate the gastric mill rhythm. Selective conjoint stimulation of MCN1 and CPN2, approximating their VCN-elicited activity patterns and firing frequencies, elicited a VCN-like gastric mill rhythm. Thus the VCN mechanosensory system elicits the gastric mill rhythm via its activation of a subset of the relevant projection neurons.
Figures


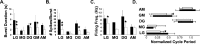



References
-
- Beenhakker MP (2004) Sensory regulation of rhythmically active neuronal networks. PhD thesis, University of Pennsylvania.
-
- Beenhakker MP, Hertzberg S, Nusbaum MP (2000) Neural network modulation by mechanosensory activation. Soc Neurosci Abstr 26: 163.2.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
