Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium
- PMID: 15282284
- PMCID: PMC5328671
- DOI: 10.1523/JNEUROSCI.4753-03.2004
Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium
Abstract
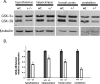
Lithium is widely used to treat bipolar disorder, but its mechanism of action in this disorder is unknown. Several molecular targets of lithium have been identified, but these putative targets have not been shown to be responsible for the behavioral effects of lithium in vivo. A robust model for the effects of chronic lithium on behavior in mice would greatly facilitate the characterization of lithium action. We describe behaviors in mice that are robustly affected by chronic lithium. Remarkably, these lithium-sensitive behaviors are also observed in mice lacking one copy of the gene encoding glycogen synthase kinase-3beta (Gsk-3beta), a well established direct target of lithium. In addition, chronic lithium induces molecular changes consistent with inhibition of GSK-3 within regions of the brain that are paralleled in Gsk-3beta+/- heterozygous mice. We also show that lithium therapy activates Wnt signaling in vivo, as measured by increased Wnt-dependent gene expression in the amygdala, hippocampus, and hypothalamus. These observations support a central role for GSK-3beta in mediating behavioral responses to lithium.
Figures






Comment in
-
There's more to lithium than Nirvana.Nat Rev Mol Cell Biol. 2013 Aug;14(8):466. doi: 10.1038/nrm3628. Epub 2013 Jul 10. Nat Rev Mol Cell Biol. 2013. PMID: 23839580 No abstract available.
References
-
- Belmaker RH, Kofman O (1990) Lithium research: state of the art. Biol Psychiatry 27: 1279-1281. - PubMed
-
- Berridge MJ, Downes CP, Hanley MR (1989) Neural and developmental actions of lithium: a unifying hypothesis. Cell 59: 411-419. - PubMed
-
- Burden SJ (2000) Wnts as retrograde signals for axon and growth cone differentiation. Cell 100: 495-497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
