Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons
- PMID: 15282285
- PMCID: PMC6729714
- DOI: 10.1523/JNEUROSCI.5463-03.2004
Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons
Abstract
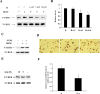
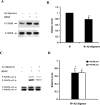
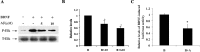
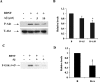
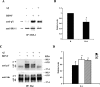
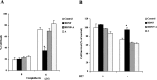
The accumulation of beta-amyloid (Abeta) is one of the etiological factors in Alzheimer's disease (AD). It has been assumed that the underlying mechanism involves a critical role of Abeta-induced neurodegeneration. However, low levels of Abeta, such as will accumulate during the course of the disease, may interfere with neuronal function via mechanisms other than those involving neurodegeneration. We have been testing, therefore, the hypothesis that Abeta at levels insufficient to cause degeneration (sublethal) may interfere with critical signal transduction processes. In cultured cortical neurons Abeta at sublethal concentrations interferes with the brain-derived neurotrophic factor (BDNF)-induced activation of the Ras-mitogen-activated protein kinase/extracellular signal-regulated protein kinase (ERK) and phosphatidylinositol 3-kinase (PI3-K)/Akt pathways. The effect of sublethal Abeta(1-42) on BDNF signaling results in the suppression of the activation of critical transcription factor cAMP response element-binding protein and Elk-1 and cAMP response element-mediated and serum response element-mediated transcription. The site of interference with the Ras/ERK and PI3-K/Akt signaling is downstream of the TrkB receptor and involves docking proteins insulin receptor substrate-1 and Shc, which convey receptor activation to the downstream effectors. The functional consequences of Abeta interference with signaling are robust, causing increased vulnerability of neurons, abrogating BDNF protection against DNA damage- and trophic deprivation-induced apoptosis. These new findings suggest that Abeta engenders a dysfunctional encoding state in neurons and may initiate and/or contribute to cognitive deficit at an early stage of AD before or along with neuronal degeneration.
Figures




Similar articles
-
Beta -amyloid-(1-42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival Is not compromised.J Biol Chem. 2001 May 18;276(20):17301-6. doi: 10.1074/jbc.M010450200. Epub 2001 Feb 26. J Biol Chem. 2001. PMID: 11278679
-
C-terminal fragment of tetanus toxin heavy chain activates Akt and MEK/ERK signalling pathways in a Trk receptor-dependent manner in cultured cortical neurons.Biochem J. 2003 Jul 15;373(Pt 2):613-20. doi: 10.1042/BJ20030333. Biochem J. 2003. PMID: 12710887 Free PMC article.
-
Inhibition of glycogen synthase kinase-3β by Angelica sinensis extract decreases β-amyloid-induced neurotoxicity and tau phosphorylation in cultured cortical neurons.J Neurosci Res. 2011 Mar;89(3):437-47. doi: 10.1002/jnr.22563. Epub 2010 Dec 17. J Neurosci Res. 2011. PMID: 21259330
-
Medicinal Herbs and Their Derived Ingredients Protect against Cognitive Decline in In Vivo Models of Alzheimer's Disease.Int J Mol Sci. 2022 Sep 25;23(19):11311. doi: 10.3390/ijms231911311. Int J Mol Sci. 2022. PMID: 36232612 Free PMC article. Review.
-
The role of CREB and BDNF in neurobiology and treatment of Alzheimer's disease.Life Sci. 2020 Sep 15;257:118020. doi: 10.1016/j.lfs.2020.118020. Epub 2020 Jun 27. Life Sci. 2020. PMID: 32603820 Review.
Cited by
-
Elk-1 a transcription factor with multiple facets in the brain.Front Neurosci. 2011 Mar 16;5:35. doi: 10.3389/fnins.2011.00035. eCollection 2011. Front Neurosci. 2011. PMID: 21441990 Free PMC article.
-
Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits.PLoS One. 2014 Nov 12;9(11):e111898. doi: 10.1371/journal.pone.0111898. eCollection 2014. PLoS One. 2014. PMID: 25390368 Free PMC article.
-
Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies.Int J Mol Sci. 2017 Jan 28;18(2):268. doi: 10.3390/ijms18020268. Int J Mol Sci. 2017. PMID: 28134845 Free PMC article. Review.
-
Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression.Biol Psychiatry. 2010 Nov 15;68(10):885-93. doi: 10.1016/j.biopsych.2010.05.030. Epub 2010 Jul 23. Biol Psychiatry. 2010. PMID: 20655510 Free PMC article.
-
Neurobiological and therapeutic landmarks of depression associated with Alzheimer's disease dementia.Front Aging Neurosci. 2025 Jun 2;17:1584607. doi: 10.3389/fnagi.2025.1584607. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40529210 Free PMC article. Review.
References
-
- Abe K, Saito H (2000) Amyloid beta neurotoxicity not mediated by the mitogen-activated protein kinase cascade in cultured rat hippocampal and cortical neurons. Neurosci Lett 292: 1-4. - PubMed
-
- Abel T, Kandel E (1998) Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev 26: 360-378. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD (1998) The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1: 602-609. - PubMed
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms [comments]. Science 286: 1358-1362. - PubMed
-
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59-68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
