On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy
- PMID: 15282287
- PMCID: PMC6729718
- DOI: 10.1523/JNEUROSCI.1449-04.2004
On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy
Abstract
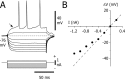
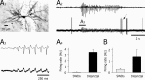
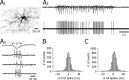
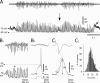
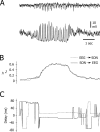
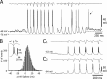
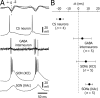
Absence seizures are characterized by impairment of consciousness associated with widespread bilaterally synchronous spike-and-wave discharges (SWDs) in the electroencephalogram (EEG), which reflect highly synchronized oscillations in thalamocortical networks. Although recent pharmacological studies suggest that the basal ganglia could provide a remote control system for absence seizures, the mechanisms of propagation of epileptic discharges in these subcortical nuclei remain unknown. In the present study, we provide the first description of the electrical events in the corticostriatal pathway during spontaneous SWDs in the genetic absence epilepsy rats from Strasbourg (GAERS), a genetic model of absence epilepsy. In corticostriatal neurons, the SWDs were associated with suprathreshold rhythmic depolarizations in-phase with local EEG spikes. Consistent with this synchronized firing in their excitatory cortical afferents, striatal output neurons (SONs) exhibited, during SWDs, large-amplitude rhythmic synaptic depolarizations. However, SONs did not discharge during SWDs. Instead, the rhythmic synaptic excitation of SONs was shunted by a Cl(-)-dependent increase in membrane conductance that was temporally correlated with bursts of action potentials in striatal GABAergic interneurons. The reduced SON excitability accompanying absence seizures may participate in the control of SWDs by affecting the flow of cortical information within the basal ganglia circuits.
Figures


References
-
- Chang HT, Wilson CJ, Kitai ST (1982) A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol 208: 107-126. - PubMed
-
- Charpier S, Lereshe N, Deniau J-M, Mahon S, Hughes SW, Crunelli V (1999) On the putative contribution of GABAB receptors to the electrical events occurring during spontaneous spike and waves discharges. Neuropharmacology 38: 1699-1706. - PubMed
-
- Connors BW, Gutnick MJ (1990) Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99-104. - PubMed
-
- Cowan RL, Wilson CJ (1994) Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol 71: 17-32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
