Regulation of an Aplysia bag-cell neuron cation channel by closely associated protein kinase A and a protein phosphatase
- PMID: 15282289
- PMCID: PMC2873327
- DOI: 10.1523/JNEUROSCI.1694-04.2004
Regulation of an Aplysia bag-cell neuron cation channel by closely associated protein kinase A and a protein phosphatase
Abstract
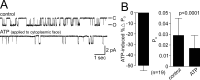
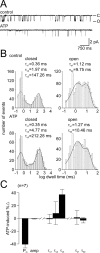
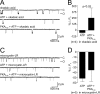
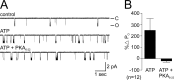
Ion channel regulation by closely associated kinases or phosphatases has emerged as a key mechanism for orchestrating neuromodulation. An exemplary case is the nonselective cation channel that drives the afterdischarge in Aplysia bag cell neurons. Initial studies showed that this channel is modulated by both a closely associated PKC and a serine/threonine protein phosphatase (PP). In excised, inside-out patches, the addition of ATP (a phosphate source) increases open probability (P(O)) through PKC, and this is reversed by the PP. Previous work also reported that, in certain cases, ATP can decrease cation channel P(O). The present study characterizes and provides a mechanism for this decreased P(O) ATP response. The kinetic change for channels inhibited by ATP was identical to the previously reported effect of exogenously applied protein kinase A (PKA) (i.e., a lengthening of the third closed-state time constant). The decreased P(O) ATP response was blocked by the PKA inhibitor peptide PKA(6-22), and its reversal was prevented by the PP inhibitor microcystin-LR. Furthermore, PKA(6-22) did not alter the increased P(O) ATP response. This suggests that both PKA and a PP are closely associated with these cation channels, but PKA and PKC are not simultaneously targeted. After an afterdischarge, the bag cell neurons are refractory and fail to respond to subsequent stimulation. The association of PKA with the cation channel may contribute to this decrease in excitability. Altering the constituents of a regulatory complex, such as exchanging PKA for PKC, may represent a general mechanism to precisely control ion channel function and excitability.
Figures




Similar articles
-
A Closely Associated Phospholipase C Regulates Cation Channel Function through Phosphoinositide Hydrolysis.J Neurosci. 2018 Aug 29;38(35):7622-7634. doi: 10.1523/JNEUROSCI.0586-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037836 Free PMC article.
-
Regulation of cation channel voltage and Ca2+ dependence by multiple modulators.J Neurophysiol. 2009 Jul;102(1):259-71. doi: 10.1152/jn.00065.2009. Epub 2009 Apr 22. J Neurophysiol. 2009. PMID: 19386758
-
Protein kinase modulation of a neuronal cation channel requires protein-protein interactions mediated by an Src homology 3 domain.J Neurosci. 2002 Jan 1;22(1):1-9. doi: 10.1523/JNEUROSCI.22-01-00001.2002. J Neurosci. 2002. PMID: 11756482 Free PMC article.
-
Modulation of a calcium-sensitive nonspecific cation channel by closely associated protein kinase and phosphatase activities.Proc Natl Acad Sci U S A. 1998 Sep 1;95(18):10938-43. doi: 10.1073/pnas.95.18.10938. Proc Natl Acad Sci U S A. 1998. PMID: 9724808 Free PMC article.
-
Supramolecular assemblies and localized regulation of voltage-gated ion channels.Physiol Rev. 2009 Apr;89(2):411-52. doi: 10.1152/physrev.00029.2007. Physiol Rev. 2009. PMID: 19342611 Free PMC article. Review.
Cited by
-
Isolation of sensory neurons of Aplysia californica for patch clamp recordings of glutamatergic currents.J Vis Exp. 2013 Jul 10;(77):e50543. doi: 10.3791/50543. J Vis Exp. 2013. PMID: 23892672 Free PMC article.
-
Calcium-dependent phosphorylation regulates neuronal stability and plasticity in a highly precise pacemaker nucleus.J Neurophysiol. 2011 Jul;106(1):319-31. doi: 10.1152/jn.00741.2010. Epub 2011 Apr 27. J Neurophysiol. 2011. PMID: 21525377 Free PMC article.
-
Cholinergic depolarization recruits a persistent Ca2+ current in Aplysia bag cell neurons.J Neurophysiol. 2023 May 1;129(5):1045-1060. doi: 10.1152/jn.00429.2022. Epub 2023 Mar 29. J Neurophysiol. 2023. PMID: 36988203 Free PMC article.
-
A Closely Associated Phospholipase C Regulates Cation Channel Function through Phosphoinositide Hydrolysis.J Neurosci. 2018 Aug 29;38(35):7622-7634. doi: 10.1523/JNEUROSCI.0586-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037836 Free PMC article.
-
Association/dissociation of a channel-kinase complex underlies state-dependent modulation.J Neurosci. 2005 Aug 31;25(35):8037-47. doi: 10.1523/JNEUROSCI.1903-05.2005. J Neurosci. 2005. PMID: 16135761 Free PMC article.
References
-
- Alonso A, Llinas RR (1989) Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342: 175-177. - PubMed
-
- Ashby CD, Walsh DA (1972) Characterization of the interaction of a protein inhibitor with adenosine 3′,5′-monophosphate-dependent protein kinases. J Biol Chem 247: 6637-6642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
