Upstream regulatory role for XIAP in receptor-mediated apoptosis
- PMID: 15282301
- PMCID: PMC479745
- DOI: 10.1128/MCB.24.16.7003-7014.2004
Upstream regulatory role for XIAP in receptor-mediated apoptosis
Abstract
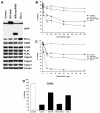
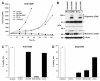
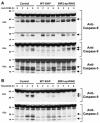
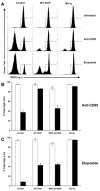
X-linked inhibitor of apoptosis (XIAP) is an endogenous inhibitor of cell death that functions by suppressing caspases 3, 7, and 9. Here we describe the establishment of Jurkat-derived cell lines stably overexpressing either full-length XIAP or a truncation mutant of XIAP that can only inhibit caspase 9. Characterization of these cell lines revealed that following CD95 activation full-length XIAP supported both short- and long-term survival as well as proliferative capacity, in contrast to the truncation mutant but similar to Bcl-x(L). Full-length XIAP was also able to inhibit CD95-mediated caspase 3 processing and activation, the mitochondrial release of cytochrome c and Smac/DIABLO, and the loss of mitochondrial membrane potential, whereas the XIAP truncation mutant failed to prevent any of these cell death events. Finally, suppression of XIAP levels by RNA interference sensitized Bcl-x(L)-overexpressing cells to death receptor-induced apoptosis. These data demonstrate for the first time that full-length XIAP inhibits caspase activation required for mitochondrial amplification of death receptor signals and that, by acting upstream of mitochondrial activation, XIAP supports the long-term proliferative capacity of cells following CD95 stimulation.
Figures



Similar articles
-
Effects of antioxidants and caspase-3 inhibitor on the phenylethyl isothiocyanate-induced apoptotic signaling pathways in human PLC/PRF/5 cells.Eur J Pharmacol. 2005 Aug 22;518(2-3):96-106. doi: 10.1016/j.ejphar.2005.06.021. Eur J Pharmacol. 2005. PMID: 16054126
-
Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells.Oncogene. 2004 Jun 3;23(26):4523-35. doi: 10.1038/sj.onc.1207594. Oncogene. 2004. PMID: 15064710
-
X-linked inhibitor regulating TRAIL-induced apoptosis in chemoresistant human primary glioblastoma cells.Clin Invest Med. 2003 Oct;26(5):231-42. Clin Invest Med. 2003. PMID: 14596484
-
Death receptors leave a caspase footprint that Smacs of XIAP.Cell Death Differ. 2003 Jan;10(1):4-6. doi: 10.1038/sj.cdd.4401176. Cell Death Differ. 2003. PMID: 12655287 Review. No abstract available.
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
Cited by
-
Xaf1 can cooperate with TNFalpha in the induction of apoptosis, independently of interaction with XIAP.Mol Cell Biochem. 2006 Jun;286(1-2):67-76. doi: 10.1007/s11010-005-9094-2. Epub 2006 Jan 24. Mol Cell Biochem. 2006. PMID: 16432762
-
Caspase-independent cell death: leaving the set without the final cut.Oncogene. 2008 Oct 27;27(50):6452-61. doi: 10.1038/onc.2008.311. Oncogene. 2008. PMID: 18955972 Free PMC article. Review.
-
Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization.Cell. 2010 May 28;141(5):859-71. doi: 10.1016/j.cell.2010.03.053. Cell. 2010. PMID: 20510932 Free PMC article.
-
Systems analysis of apoptosis protein expression allows the case-specific prediction of cell death responsiveness of melanoma cells.Cell Death Differ. 2013 Nov;20(11):1521-31. doi: 10.1038/cdd.2013.106. Epub 2013 Aug 9. Cell Death Differ. 2013. PMID: 23933815 Free PMC article.
-
XIAP inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells.Oncotarget. 2014 Jun 30;5(12):4337-46. doi: 10.18632/oncotarget.2016. Oncotarget. 2014. PMID: 24952669 Free PMC article.
References
-
- Acehan, D., X. Jiang, D. G. Morgan, J. E. Heuser, X. Wang, and C. W. Akey. 2002. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9:423-432. - PubMed
-
- Amarante-Mendes, G. P., D. M. Finucane, S. J. Martin, T. G. Cotter, G. S. Salvesen, and D. R. Green. 1998. Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 5:298-306. - PubMed
-
- Birkey Reffey, S., J. U. Wurthner, W. T. Parks, A. B. Roberts, and C. S. Duckett. 2001. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem. 276:26542-26549. - PubMed
-
- Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
