Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo
- PMID: 15282303
- PMCID: PMC479722
- DOI: 10.1128/MCB.24.16.7024-7031.2004
Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo
Abstract
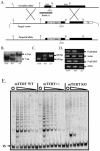
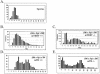
Telomerase consists of two essential components, the telomerase RNA template (TR) and telomerase reverse transcriptase (TERT). The haplo-insufficiency of TR was recently shown to cause one form of human dyskeratosis congenita, an inherited disease marked by abnormal telomere shortening. Consistent with this finding, we recently reported that mice heterozygous for inactivation of mouse TR exhibit a similar haplo-insufficiency and are deficient in the ability to elongate telomeres in vivo. To further assess the genetic regulation of telomerase activity, we have compared the abilities of TR-deficient and TERT-deficient mice to maintain or elongate telomeres in interspecies crosses. Homozygous TERT knockout mice had no telomerase activity and failed to maintain telomere length. In contrast, TERT(+/-) heterozygotes had no detectable defect in telomere elongation compared to wild-type controls, whereas TR(+/-) heterozygotes were deficient in telomere elongation. Levels of TERT mRNA in heterozygous mice were one-third to one-half the levels expressed in wild-type mice, similar to the reductions in telomerase RNA observed in TR heterozygotes. These findings indicate that both TR and TERT are essential for telomere maintenance and elongation but that gene copy number and transcriptional regulation of TR, but not TERT, are limiting for telomerase activity under the in vivo conditions analyzed.
Figures




Similar articles
-
Phenotypes in mTERT⁺/⁻ and mTERT⁻/⁻ mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase.Mol Cell Biol. 2011 Jun;31(12):2369-79. doi: 10.1128/MCB.05312-11. Epub 2011 Apr 4. Mol Cell Biol. 2011. PMID: 21464209 Free PMC article.
-
The structure and function of telomerase reverse transcriptase.Annu Rev Biochem. 2006;75:493-517. doi: 10.1146/annurev.biochem.75.103004.142412. Annu Rev Biochem. 2006. PMID: 16756500 Review.
-
Short telomeres, even in the presence of telomerase, limit tissue renewal capacity.Cell. 2005 Dec 16;123(6):1121-31. doi: 10.1016/j.cell.2005.11.020. Cell. 2005. PMID: 16360040
-
Ku70 prevents genome instability resulting from heterozygosity of the telomerase RNA component in a vertebrate tumour line.DNA Repair (Amst). 2008 May 3;7(5):713-24. doi: 10.1016/j.dnarep.2008.01.008. Epub 2008 Mar 4. DNA Repair (Amst). 2008. PMID: 18308646
-
Telomerase RNA levels limit the telomere length equilibrium.Cold Spring Harb Symp Quant Biol. 2006;71:225-9. doi: 10.1101/sqb.2006.71.063. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381301 Review.
Cited by
-
Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy.Kidney Int. 2015 Jul;88(1):85-94. doi: 10.1038/ki.2015.69. Epub 2015 Mar 11. Kidney Int. 2015. PMID: 25760322 Free PMC article.
-
Telomerase and telomere length in pulmonary fibrosis.Am J Respir Cell Mol Biol. 2013 Aug;49(2):260-8. doi: 10.1165/rcmb.2012-0514OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526226 Free PMC article. Clinical Trial.
-
Making the most of a little: dosage effects in eukaryotic telomere length maintenance.Chromosome Res. 2005;13(5):493-504. doi: 10.1007/s10577-005-0994-5. Chromosome Res. 2005. PMID: 16132814 Review.
-
Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage.J Neurosci. 2007 Apr 4;27(14):3722-33. doi: 10.1523/JNEUROSCI.0590-07.2007. J Neurosci. 2007. PMID: 17409236 Free PMC article.
-
The role of telomeres in the ageing of human skin.Exp Dermatol. 2011 Apr;20(4):297-302. doi: 10.1111/j.1600-0625.2010.01242.x. Epub 2011 Mar 3. Exp Dermatol. 2011. PMID: 21371125 Free PMC article. Review.
References
-
- Artandi, S. E., S. Alson, M. K. Tietze, N. E. Sharpless, S. Ye, R. A. Greenberg, D. H. Castrillon, J. W. Horner, S. R. Weiler, R. D. Carrasco, and R. A. DePinho. 2002. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc. Natl. Acad. Sci. USA 99:8191-8196. - PMC - PubMed
-
- Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. - PubMed
-
- Blackburn, E. H., C. W. Greider, E. Henderson, M. S. Lee, J. Shampay, and D. Shippen-Lentz. 1989. Recognition and elongation of telomeres by telomerase. Genome 31:553-560. - PubMed
-
- Blasco, M. A., W. Funk, B. Villeponteau, and C. W. Greider. 1995. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269:1267-1270. - PubMed
-
- Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
