Vanin-1-/- mice exhibit a glutathione-mediated tissue resistance to oxidative stress
- PMID: 15282320
- PMCID: PMC479710
- DOI: 10.1128/MCB.24.16.7214-7224.2004
Vanin-1-/- mice exhibit a glutathione-mediated tissue resistance to oxidative stress
Abstract
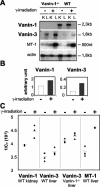
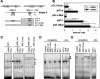
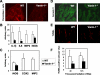
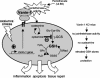
Vanin-1 is an epithelial ectoenzyme with pantetheinase activity and generating the amino-thiol cysteamine through the metabolism of pantothenic acid (vitamin B(5)). Here we show that Vanin-1(-/-) mice, which lack cysteamine in tissues, exhibit resistance to oxidative injury induced by whole-body gamma-irradiation or paraquat. This protection is correlated with reduced apoptosis and inflammation and is reversed by treating mutant animals with cystamine. The better tolerance of the Vanin-1(-/-) mice is associated with an enhanced gamma-glutamylcysteine synthetase activity in liver, probably due to the absence of cysteamine and leading to elevated stores of glutathione (GSH), the most potent cellular antioxidant. Consequently, Vanin-1(-/-) mice maintain a more reducing environment in tissue after exposure to irradiation. In normal mice, we found a stress-induced biphasic expression of Vanin-1 regulated via antioxidant response elements in its promoter region. This process should finely tune the redox environment and thus change an early inflammatory process into a late tissue repair process. We propose Vanin-1 as a key molecule to regulate the GSH-dependent response to oxidative injury in tissue at the epithelial level. Therefore, Vanin/pantetheinase inhibitors could be useful for treatment of damage due to irradiation and pro-oxidant inducers.
Figures



References
-
- Andrews, G. K. 2000. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 59:95-104. - PubMed
-
- Arudchelvan, Y., N. Tokuda, M. Tamechika, Y. H. Wang, N. Mizutani, T. Sawada, K. Yamaguchi, T. Fukumoto, and F. Shinozaki. 2000. Semiquantitative morphological analysis of stromal cells in the irradiated and recovering rat thymus. Arch. Histol. Cytol. 63:147-157. - PubMed
-
- Aurrand-Lions, M., F. Galland, H. Bazin, V. Zakharyev, B. A. Imhof, and P. Naquet. 1996. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity 5:391-405. - PubMed
-
- Chanas, S. A., Q. Jiang, M. McMahon, G. K. McWalter, L. I. McLellan, C. R. Elcombe, C. J. Henderson, C. R. Wolf, G. J. Moffat, K. Itoh, M. Yamamoto, and J. D. Hayes. 2002. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem. J. 365:405-416. - PMC - PubMed
-
- Chen, W., M. E. Frank, W. Jin, and S. M. Wahl. 2001. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14:715-725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
