Protein kinase C delta blocks immediate-early gene expression in senescent cells by inactivating serum response factor
- PMID: 15282327
- PMCID: PMC479731
- DOI: 10.1128/MCB.24.16.7298-7311.2004
Protein kinase C delta blocks immediate-early gene expression in senescent cells by inactivating serum response factor
Abstract
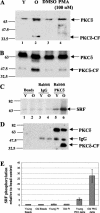
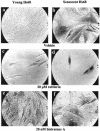
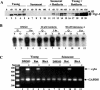
Fibroblasts lose the ability to replicate in response to growth factors and become unable to express growth-associated immediate-early genes, including c-fos and egr-1, as they become senescent. The serum response factor (SRF), a major transcriptional activator of immediate-early gene promoters, loses the ability to bind to the serum response element (SRE) and becomes hyperphosphorylated in senescent cells. We identify protein kinase C delta (PKC delta) as the kinase responsible for inactivation of SRF both in vitro and endogenously in senescent cells. This is due to a higher level of PKC delta activity as cells age, production of the PKC delta catalytic fragment, and its nuclear localization in senescent but not in low-passage-number cells. The phosphorylation of T160 of SRF by PKC delta in vitro and in vivo led to loss of SRF DNA binding activity. Both the PKC delta inhibitor rottlerin and ectopic expression of a dominant negative form of PKC delta independently restored SRE-dependent transcription and immediate-early gene expression in senescent cells. Modulation of PKC delta activity in vivo with rottlerin or bistratene A altered senescent- and young-cell morphology, respectively. These observations support the idea that the coordinate transcriptional inhibition of several growth-associated genes by PKC delta contributes to the senescent phenotype.
Figures




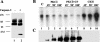


Similar articles
-
Decreased expression and activity of the immediate-early growth response (Egr-1) gene product during cellular senescence.J Cell Physiol. 1999 Apr;179(1):29-39. doi: 10.1002/(SICI)1097-4652(199904)179:1<29::AID-JCP4>3.0.CO;2-D. J Cell Physiol. 1999. PMID: 10082129
-
The polyether bistratene A activates protein kinase C-delta and induces growth arrest in HL60 cells.Biochem Biophys Res Commun. 1996 May 24;222(3):802-8. doi: 10.1006/bbrc.1996.0830. Biochem Biophys Res Commun. 1996. PMID: 8651926
-
Alpha2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts.Nucleic Acids Res. 2005 Mar 1;33(4):1337-51. doi: 10.1093/nar/gki275. Print 2005. Nucleic Acids Res. 2005. PMID: 15741186 Free PMC article.
-
Regulation of gene expression and transcription factor binding activity during cellular aging.Biol Signals. 1996 May-Jun;5(3):130-8. doi: 10.1159/000109183. Biol Signals. 1996. PMID: 8864058 Review.
-
nPKCdelta a new therapeutic marker for melanoma metastasis? (Review).Int J Mol Med. 2000 May;5(5):467-71. doi: 10.3892/ijmm.5.5.467. Int J Mol Med. 2000. PMID: 10762648 Review.
Cited by
-
Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control.Cell Div. 2007 Mar 7;2:10. doi: 10.1186/1747-1028-2-10. Cell Div. 2007. PMID: 17343761 Free PMC article.
-
High salt culture conditions inhibit serum- and NGF- but not PMA-induced Egr-1 gene transcription in rat C6 glioma cells.J Mol Neurosci. 2007;33(2):216-23. doi: 10.1007/s12031-007-9000-3. J Mol Neurosci. 2007. PMID: 17917080
-
Narrative Review: Glucocorticoids in Alcoholic Hepatitis-Benefits, Side Effects, and Mechanisms.J Xenobiot. 2022 Sep 21;12(4):266-288. doi: 10.3390/jox12040019. J Xenobiot. 2022. PMID: 36278756 Free PMC article. Review.
-
The Werner syndrome helicase protein is required for cell proliferation, immortalization, and tumorigenesis in Scaffold attachment factor B1 deficient mice.Aging (Albany NY). 2011 Mar;3(3):277-90. doi: 10.18632/aging.100300. Aging (Albany NY). 2011. PMID: 21464516 Free PMC article.
-
Histamine induces Egr-1 expression in human aortic endothelial cells via the H1 receptor-mediated protein kinase Cdelta-dependent ERK activation pathway.J Biol Chem. 2008 Oct 3;283(40):26928-36. doi: 10.1074/jbc.M803071200. Epub 2008 Aug 5. J Biol Chem. 2008. PMID: 18682391 Free PMC article.
References
-
- Abate, C., D. R. Marshak, and T. Curran. 1991. Fos is phosphorylated by p34cdc2, cAMP-dependent protein kinase and protein kinase C at multiple sites clustered within regulatory regions. Oncogene 6:2179-2185. - PubMed
-
- Bharti, A., S. K. Kraeft, M. Gounder, P. Pandey, S. Jin, Z. M. Yuan, S. P. Lees-Miller, R. Weichselbaum, D. Weaver, L. B. Chen, D. Kufe, and S. Kharbanda. 1998. Inactivation of DNA-dependent protein kinase by protein kinase Cδ: implications for apoptosis. Mol. Cell. Biol. 18:6719-6728. - PMC - PubMed
-
- Blake, R. A., P. Garcia-Paramio, P. J. Parker, and S. A. Courtneidge. 1999. Src promotes PKCδ degradation. Cell Growth Differ. 10:231-241. - PubMed
-
- Carlin, C., P. D. Phillips, K. Brooks-Frederich, B. B. Knowles, and V. J. Cristofalo. 1994. Cleavage of the epidermal growth factor receptor by a membrane-bound leupeptin-sensitive protease active in nonionic detergent lysates of senescent but not young human diploid fibroblasts. J. Cell Physiol. 160:427-434. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
