Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43
- PMID: 15282340
- PMCID: PMC519152
- DOI: 10.1091/mbc.e04-04-0324
Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43
Abstract
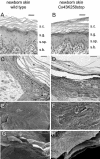
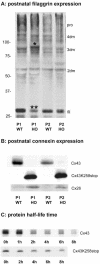
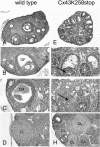
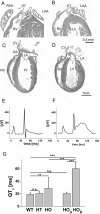
More than 97% of mice in which the C-terminal region of connexin43 (Cx43) was removed (designated as Cx43K258stop) die shortly after birth due to a defect of the epidermal barrier. The abnormal expression of Cx43K258stop protein in the uppermost layers of the epidermis seems to perturb terminal differentiation of keratinocytes. In contrast to Cx43-deficient mice, neonatal Cx43K258stop hearts show no lethal obstruction of the right ventricular outflow tract, but signs of dilatation. Electrocardiographies of neonatal hearts reveal repolarization abnormalities in 20% of homozygous Cx43K258stop animals. The very rare adult Cx43K258stop mice show a compensation of the epidermal barrier defect but persisting impairment of cardiac function in echocardiography. Female Cx43K258stop mice are infertile due to impaired folliculogenesis. Our results indicate that the C-terminally truncated Cx43K258stop mice lack essential functions of Cx43, although the truncated Cx43 protein can form open gap junctional channels.
Figures




Similar articles
-
Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants.Eur J Cell Biol. 2004 Dec;83(11-12):647-54. doi: 10.1078/0171-9335-00422. Eur J Cell Biol. 2004. PMID: 15679109 Review.
-
Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels.Basic Res Cardiol. 2013 May;108(3):348. doi: 10.1007/s00395-013-0348-y. Epub 2013 Apr 5. Basic Res Cardiol. 2013. PMID: 23558439 Free PMC article.
-
Regulation of connexin43 protein complexes by intracellular acidification.Circ Res. 2004 Feb 6;94(2):215-22. doi: 10.1161/01.RES.0000113924.06926.11. Epub 2003 Dec 29. Circ Res. 2004. PMID: 14699011
-
Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion.Mol Biol Cell. 2005 Dec;16(12):5686-98. doi: 10.1091/mbc.e05-08-0737. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195341 Free PMC article.
-
Is the junctional uncoupling elicited in rat ventricular myocytes by some dephosphorylation treatments due to changes in the phosphorylation status of Cx43?Eur Biophys J. 2004 May;33(3):201-10. doi: 10.1007/s00249-003-0381-0. Epub 2004 Jan 27. Eur Biophys J. 2004. PMID: 14745523 Review.
Cited by
-
Gap junctions mediate discrete regulatory steps during fly spermatogenesis.PLoS Genet. 2022 Sep 29;18(9):e1010417. doi: 10.1371/journal.pgen.1010417. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36174062 Free PMC article.
-
Connexins and Pannexins in Bone and Skeletal Muscle.Curr Osteoporos Rep. 2017 Aug;15(4):326-334. doi: 10.1007/s11914-017-0374-z. Curr Osteoporos Rep. 2017. PMID: 28647887 Free PMC article. Review.
-
Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects.Hum Mutat. 2011 Apr;32(4):456-66. doi: 10.1002/humu.21472. Hum Mutat. 2011. PMID: 21305658 Free PMC article.
-
The molecular role of connexin 43 in human trophoblast cell fusion.Biol Reprod. 2012 Apr 19;86(4):115. doi: 10.1095/biolreprod.111.096925. Print 2012 Apr. Biol Reprod. 2012. PMID: 22238282 Free PMC article.
-
Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation.Physiology (Bethesda). 2013 Mar;28(2):93-116. doi: 10.1152/physiol.00038.2012. Physiology (Bethesda). 2013. PMID: 23455769 Free PMC article. Review.
References
-
- Ackert, C.L., Gittens, J.E., O'Brien, M.J., Eppig, J.J., and Kidder, G.M. (2001). Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 233, 258-270. - PubMed
-
- Bakirtzis, G., et al. (2003). Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum. Mol. Genet. 12, 1737-1744. - PubMed
-
- Barker, R.J., Price, R.L., and Gourdie, R.G. (2002). Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ. Res. 90, 317-324. - PubMed
-
- Cartlidge, P. (2000). The epidermal barrier. Semin. Neonatol. 5, 273-280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

