Folate modulates Hox gene-controlled skeletal phenotypes
- PMID: 15282741
- PMCID: PMC3938166
- DOI: 10.1002/gene.20036
Folate modulates Hox gene-controlled skeletal phenotypes
Abstract
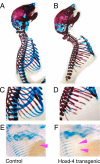
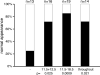
Hox genes are well-known regulators of pattern formation and cell differentiation in the developing vertebrate skeleton. Although skeletal variations are not uncommon in humans few mutations in human HOX genes have been described. If such mutations are compatible with life, there may be physiological modifiers for the manifestation of Hox gene-controlled phenotypes, masking underlying mutations. Here we present evidence that the essential nutrient folate modulates genetically induced skeletal defects in Hoxd4 transgenic mice. We also show that chondrocytes require folate for growth and differentiation and that they express folate transport genes, providing evidence for a direct effect of folate on skeletal cells. To our knowledge, this is the first report of nutritional influence on Hox gene-controlled phenotypes, and implicates gene-environment interactions as important modifiers of Hox gene function. Taken together, our results demonstrate a beneficial effect of folate on skeletal development that may also be relevant to disorders and variations of the human skeleton.
Copyright 2004 Wiley-Liss, Inc.
Figures


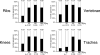
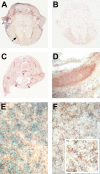
Similar articles
-
Expression of folate pathway genes in the cartilage of Hoxd4 and Hoxc8 transgenic mice.Birth Defects Res A Clin Mol Teratol. 2006 Apr;76(4):216-29. doi: 10.1002/bdra.20245. Birth Defects Res A Clin Mol Teratol. 2006. PMID: 16586448 Free PMC article.
-
Expressing Hoxa2 across the entire endochondral skeleton alters the shape of the skeletal template in a spatially restricted fashion.Differentiation. 2010 Mar;79(3):194-202. doi: 10.1016/j.diff.2009.11.004. Epub 2010 Jan 14. Differentiation. 2010. PMID: 20034726
-
Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos.Dev Cell. 2009 Oct;17(4):516-26. doi: 10.1016/j.devcel.2009.08.010. Dev Cell. 2009. PMID: 19853565
-
Hox genes and limb musculoskeletal development.Curr Osteoporos Rep. 2014 Dec;12(4):420-7. doi: 10.1007/s11914-014-0241-0. Curr Osteoporos Rep. 2014. PMID: 25266923 Free PMC article. Review.
-
Coupling the roles of Hox genes to regulatory networks patterning cranial neural crest.Dev Biol. 2018 Dec 1;444 Suppl 1:S67-S78. doi: 10.1016/j.ydbio.2018.03.016. Epub 2018 Mar 20. Dev Biol. 2018. PMID: 29571614 Review.
Cited by
-
Developmental Patterning as a Quantitative Trait: Genetic Modulation of the Hoxb6 Mutant Skeletal Phenotype.PLoS One. 2016 Jan 22;11(1):e0146019. doi: 10.1371/journal.pone.0146019. eCollection 2016. PLoS One. 2016. PMID: 26800342 Free PMC article.
-
Microarray Analysis of Defective Cartilage in Hoxc8- and Hoxd4-Transgenic Mice.Cartilage. 2010 Jul;1(3):217-32. doi: 10.1177/1947603510363005. Cartilage. 2010. PMID: 26069554 Free PMC article.
-
Genetic and epigenomic footprints of folate.Prog Mol Biol Transl Sci. 2012;108:129-58. doi: 10.1016/B978-0-12-398397-8.00006-X. Prog Mol Biol Transl Sci. 2012. PMID: 22656376 Free PMC article. Review.
-
Degradome expression profiling in human articular cartilage.Arthritis Res Ther. 2009;11(3):R96. doi: 10.1186/ar2741. Epub 2009 Jun 23. Arthritis Res Ther. 2009. PMID: 19549314 Free PMC article.
-
Morpholino-mediated knockdown in primary chondrocytes implicates Hoxc8 in regulation of cell cycle progression.Bone. 2009 Apr;44(4):708-16. doi: 10.1016/j.bone.2008.10.057. Epub 2008 Nov 21. Bone. 2009. PMID: 19071237 Free PMC article.
References
-
- Akarsu AN, Stoilov I, Yilmaz E, Sayli BS, Sarfarazi M. Genomic structure of HOXD13 gene: a nine polyalanine duplication causes synpolydactyly in two unrelated families. Hum Mol Genet. 1996;5:945–952. - PubMed
-
- Antony AC. Folate Receptors. Annu Rev Nutr. 1996;16:501–521. - PubMed
-
- Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–293. - PubMed
-
- Berry RJ, Li Z. Folic acid alone prevents neural tube defects: evidence from the China study. Epidemiology. 2002;13:114–116. - PubMed
-
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

