Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism
- PMID: 15283675
- PMCID: PMC1134042
- DOI: 10.1042/BJ20040634
Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism
Abstract
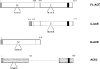
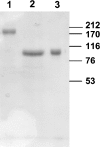
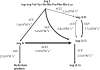
In the RAS (renin-angiotensin system), Ang I (angiotensin I) is cleaved by ACE (angiotensin-converting enzyme) to form Ang II (angiotensin II), which has effects on blood pressure, fluid and electrolyte homoeostasis. We have examined the kinetics of angiotensin peptide cleavage by full-length human ACE, the separate N- and C-domains of ACE, the homologue of ACE, ACE2, and NEP (neprilysin). The activity of the enzyme preparations was determined by active-site titrations using competitive tight-binding inhibitors and fluorogenic substrates. Ang I was effectively cleaved by NEP to Ang (1-7) (kcat/K(m) of 6.2x10(5) M(-1) x s(-1)), but was a poor substrate for ACE2 (kcat/K(m) of 3.3x10(4) M(-1) x s(-1)). Ang (1-9) was a better substrate for NEP than ACE (kcat/K(m) of 3.7x10(5) M(-1) x s(-1) compared with kcat/K(m) of 6.8x10(4) M(-1) x s(-1)). Ang II was cleaved efficiently by ACE2 to Ang (1-7) (kcat/K(m) of 2.2x10(6) M(-1) x s(-1)) and was cleaved by NEP (kcat/K(m) of 2.2x10(5) M(-1) x s(-1)) to several degradation products. In contrast with a previous report, Ang (1-7), like Ang I and Ang (1-9), was cleaved with a similar efficiency by both the N- and C-domains of ACE (kcat/K(m) of 3.6x10(5) M(-1) x s(-1) compared with kcat/K(m) of 3.3x10(5) M(-1) x s(-1)). The two active sites of ACE exhibited negative co-operativity when either Ang I or Ang (1-7) was the substrate. In addition, a range of ACE inhibitors failed to inhibit ACE2. These kinetic data highlight that the flux of peptides through the RAS is complex, with the levels of ACE, ACE2 and NEP dictating whether vasoconstriction or vasodilation will predominate.
Figures
References
-
- Soubrier F., Hubert C., Testut P., Nadaud S., Alhenc-Gelas F., Corvol P. Molecular biology of the angiotensin I converting enzyme: I. biochemistry and structure of the gene. J. Hypertens. 1993;11:471–476. - PubMed
-
- Rice G. I., Foy C. A., Grant P. J. Angiotensin converting enzyme and angiotensin II type 1-receptor gene polymorphisms and risk of ischaemic heart disease. Cardiovasc. Res. 1999;41:746–753. - PubMed
-
- Dzau V. J. Cell biology and genetics of angiotensin in cardiovascular disease. J. Hypertens. 1994;12:S3–S10. - PubMed
-
- Brown N. J., Vaughan D. E. The renin–angiotensin and fibrinolytic systems: co-conspirators in the pathogenesis of ischemic cardiovascular disease. Trends Cardiovasc. Med. 1996;6:239–243. - PubMed
-
- Fluharty S. J., Reagan L. P., Yee D. K. The angiotensin type 1 and type 2 receptor families: siblings or cousins? In: Mukhopadhyay A. K., Raizada M. K., editors. Tissue Renin–Angiotensin Systems. New York: Plenum Press; 1995. pp. 193–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

