The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep
- PMID: 15284345
- PMCID: PMC1665213
- DOI: 10.1113/jphysiol.2004.072371
The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep
Abstract
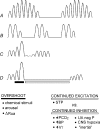
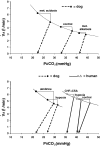
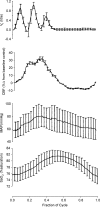
Sleep unmasks a highly sensitive hypocapnia-induced apnoeic threshold, whereby apnoea is initiated by small transient reductions in arterial CO(2) pressure (P(aCO(2))) below eupnoea and respiratory rhythm is not restored until P(aCO(2)) has risen significantly above eupnoeic levels. We propose that the 'CO(2) reserve' (i.e. the difference in P(aCO(2)) between eupnoea and the apnoeic threshold (AT)), when combined with 'plant gain' (or the ventilatory increase required for a given reduction in P(aCO(2))) and 'controller gain' (ventilatory responsiveness to CO(2) above eupnoea) are the key determinants of breathing instability in sleep. The CO(2) reserve varies inversely with both plant gain and the slope of the ventilatory response to reduced CO(2) below eupnoea; it is highly labile in non-random eye movement (NREM) sleep. With many types of increases or decreases in background ventilatory drive and P(aCO(2)), the slope of the ventilatory response to reduced P(aCO(2)) below eupnoea remains unchanged from control. Thus, the CO(2) reserve varies inversely with plant gain, i.e. it is widened with hyperventilation and narrowed with hypoventilation, regardless of the stimulus and whether it acts primarily at the peripheral or central chemoreceptors. However, there are notable exceptions, such as hypoxia, heart failure, or increased pulmonary vascular pressures, which all increase the slope of the CO(2) response below eupnoea and narrow the CO(2) reserve despite an accompanying hyperventilation and reduced plant gain. Finally, we review growing evidence that chemoreceptor-induced instability in respiratory motor output during sleep contributes significantly to the major clinical problem of cyclical obstructive sleep apnoea.
Figures

Similar articles
-
Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep.Am J Respir Crit Care Med. 2002 May 1;165(9):1251-60. doi: 10.1164/rccm.2110041. Am J Respir Crit Care Med. 2002. PMID: 11991874
-
Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep.J Appl Physiol (1985). 2010 Nov;109(5):1378-83. doi: 10.1152/japplphysiol.00453.2010. Epub 2010 Aug 19. J Appl Physiol (1985). 2010. PMID: 20724559 Free PMC article. Clinical Trial.
-
Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure.Am J Respir Crit Care Med. 2010 Jan 15;181(2):189-93. doi: 10.1164/rccm.200810-1658OC. Epub 2009 Sep 17. Am J Respir Crit Care Med. 2010. PMID: 19762565 Free PMC article.
-
Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation.Am J Physiol Regul Integr Comp Physiol. 2009 May;296(5):R1473-95. doi: 10.1152/ajpregu.91008.2008. Epub 2009 Feb 11. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19211719 Review.
-
The essential role of carotid body chemoreceptors in sleep apnea.Can J Physiol Pharmacol. 2003 Aug;81(8):774-9. doi: 10.1139/y03-056. Can J Physiol Pharmacol. 2003. PMID: 12897806 Review.
Cited by
-
Inherent vs. Induced Loop Gain Abnormalities in Obstructive Sleep Apnea.Front Neurol. 2018 Nov 2;9:896. doi: 10.3389/fneur.2018.00896. eCollection 2018. Front Neurol. 2018. PMID: 30450076 Free PMC article. Review.
-
A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude.J Physiol. 2009 Feb 15;587(Pt 4):883-96. doi: 10.1113/jphysiol.2008.160689. Epub 2008 Dec 22. J Physiol. 2009. PMID: 19103684 Free PMC article.
-
Influence of cerebral blood flow on breathing stability.J Appl Physiol (1985). 2009 Mar;106(3):850-6. doi: 10.1152/japplphysiol.90914.2008. Epub 2008 Dec 31. J Appl Physiol (1985). 2009. PMID: 19118158 Free PMC article.
-
Buspirone decreases susceptibility to hypocapnic central sleep apnea in chronic SCI patients.J Appl Physiol (1985). 2020 Oct 1;129(4):675-682. doi: 10.1152/japplphysiol.00435.2020. Epub 2020 Aug 20. J Appl Physiol (1985). 2020. PMID: 32816639 Free PMC article. Clinical Trial.
-
Carbon dioxide and MAPK signalling: towards therapy for inflammation.Cell Commun Signal. 2023 Oct 10;21(1):280. doi: 10.1186/s12964-023-01306-x. Cell Commun Signal. 2023. PMID: 37817178 Free PMC article. Review.
References
-
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. - PubMed
-
- Batsel HL. Activity of bulbar respiratory neurons during passive hyperventilation. Exp Neurol. 1967;19:357–374. - PubMed
-
- Berssenbrugge AD, Dempsey JA, Skatrud JB. Effects of sleep state on ventilatory acclimatization to hypoxia in humans. J Appl Physiol. 1984;57:1089–1096. - PubMed
-
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

