Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42
- PMID: 15285798
- PMCID: PMC483057
- DOI: 10.1186/1742-2094-1-4
Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42
Abstract
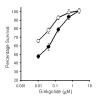
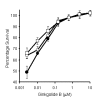
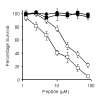
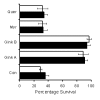
BACKGROUND: Neuronal loss in Alzheimer's or prion diseases is preceded by the accumulation of fibrillar aggregates of toxic proteins (amyloid-beta1-42 or the prion protein). Since some epidemiological studies have demonstrated that the EGb 761 extract, from the leaves of the Ginkgo biloba tree, has a beneficial effect on Alzheimer's disease, the effect of some of the major components of the EGb 761 extract on neuronal responses to amyloid-beta1-42, or to a synthetic miniprion (sPrP106), were investigated. METHODS: Components of the EGb 761 extract were tested in 2 models of neurodegeneration. SH-SY5Y neuroblastoma cells were pre-treated with ginkgolides A or B, quercetin or myricetin, and incubated with amyloid-beta1-42, sPrP106, or other neurotoxins. After 24 hours neuronal survival and the production of prostaglandin E2 that is closely associated with neuronal death was measured. In primary cortical neurons apoptosis (caspase-3) in response to amyloid-beta1-42 or sPrP106 was measured, and in co-cultures the effects of the ginkgolides on the killing of amyloid-beta1-42 or sPrP106 damaged neurons by microglia was tested. RESULTS: Neurons treated with ginkgolides A or B were resistant to amyloid-beta1-42 or sPrP106. Ginkgolide-treated cells were also resistant to platelet activating factor or arachidonic acid, but remained susceptible to hydrogen peroxide or staurosporine. The ginkgolides reduced the production of prostaglandin E2 in response to amyloid-beta1-42 or sPrP106. In primary cortical neurons, the ginkgolides reduced caspase-3 responses to amyloid-beta1-42 or sPrP106, and in co-culture studies the ginkgolides reduced the killing of amyloid-beta1-42 or sPrP106 damaged neurons by microglia. CONCLUSION: Nanomolar concentrations of the ginkgolides protect neurons against the otherwise toxic effects of amyloid-beta1-42 or sPrP106. The ginkgolides also prevented the neurotoxicity of platelet activating factor and reduced the production of prostaglandin E2 in response to platelet activating factor, amyloid-beta1-42 or sPrP106. These results are compatible with prior reports that ginkgolides inhibit platelet-activating factor, and that platelet-activating factor antagonists block the toxicity of amyloid-beta1-42 or sPrP106. The results presented here suggest that platelet-activating factor antagonists such as the ginkgolides may be relevant treatments for prion or Alzheimer's diseases.
Figures
Similar articles
-
Ginkgolides protect against amyloid-beta1-42-mediated synapse damage in vitro.Mol Neurodegener. 2008 Jan 7;3:1. doi: 10.1186/1750-1326-3-1. Mol Neurodegener. 2008. PMID: 18179689 Free PMC article.
-
The role of platelet activating factor in prion and amyloid-beta neurotoxicity.Neuroreport. 2004 Mar 1;15(3):509-13. doi: 10.1097/00001756-200403010-00025. Neuroreport. 2004. PMID: 15094513
-
Role of glycosylphosphatidylinositols in the activation of phospholipase A2 and the neurotoxicity of prions.J Gen Virol. 2004 Dec;85(Pt 12):3797-3804. doi: 10.1099/vir.0.80366-0. J Gen Virol. 2004. PMID: 15557253
-
Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease.Pharmacopsychiatry. 2003 Jun;36 Suppl 1:S8-14. doi: 10.1055/s-2003-40454. Pharmacopsychiatry. 2003. PMID: 13130383 Review.
-
[Ginkgo biloba extract (EGb 761). State of knowledge in the dawn of the year 2000].Ann Pharm Fr. 1999 Jul;57 Suppl 1:1S8-88. Ann Pharm Fr. 1999. PMID: 10481350 Review. French.
Cited by
-
Protection against beta-amyloid induced abnormal synaptic function and cell death by Ginkgolide J.Neurobiol Aging. 2009 Feb;30(2):257-65. doi: 10.1016/j.neurobiolaging.2007.05.025. Epub 2007 Jul 20. Neurobiol Aging. 2009. PMID: 17640772 Free PMC article.
-
Squalestatin alters the intracellular trafficking of a neurotoxic prion peptide.BMC Neurosci. 2007 Nov 22;8:99. doi: 10.1186/1471-2202-8-99. BMC Neurosci. 2007. PMID: 18034899 Free PMC article.
-
Ginkgolides protect against amyloid-beta1-42-mediated synapse damage in vitro.Mol Neurodegener. 2008 Jan 7;3:1. doi: 10.1186/1750-1326-3-1. Mol Neurodegener. 2008. PMID: 18179689 Free PMC article.
-
Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans.Neurochem Res. 2012 Jan;37(1):40-8. doi: 10.1007/s11064-011-0580-5. Epub 2011 Aug 21. Neurochem Res. 2012. PMID: 21858698
-
The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice.Neuropharmacology. 2015 Jun;93:134-45. doi: 10.1016/j.neuropharm.2015.01.027. Epub 2015 Feb 7. Neuropharmacology. 2015. PMID: 25666032 Free PMC article.
References
-
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989;245:417–420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

