Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide
- PMID: 15285800
- PMCID: PMC483052
- DOI: 10.1186/1742-2094-1-2
Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide
Abstract
BACKGROUND: Roles for excitotoxicity and inflammation in Alzheimer's disease have been hypothesized. Proinflammatory stimuli, including amyloid beta-peptide (Abeta), elicit a release of glutamate from microglia. We tested the possibility that a coagonist at the NMDA class of glutamate receptors, D-serine, could respond similarly. METHODS: Cultured microglial cells were exposed to Abeta. The culture medium was assayed for levels of D-serine by HPLC and for effects on calcium and survival on primary cultures of rat hippocampal neurons. Microglial cell lysates were examined for the levels of mRNA and protein for serine racemase, the enzyme that forms D-serine from L-serine. The racemase mRNA was also assayed in Alzheimer hippocampus and age-matched controls. A microglial cell line was transfected with a luciferase reporter construct driven by the putative regulatory region of human serine racemase. RESULTS: Conditioned medium from Abeta-treated microglia contained elevated levels of D-serine. Bioassays of hippocampal neurons with the microglia-conditioned medium indicated that Abeta elevated a NMDA receptor agonist that was sensitive to an antagonist of the D-serine/glycine site (5,7-dicholorokynurenic acid; DCKA) and to enzymatic degradation of D-amino acids by D-amino acid oxidase (DAAOx). In the microglia, Abeta elevated steady-state levels of dimeric serine racemase, the apparent active form of the enzyme. Promoter-reporter and mRNA analyses suggest that serine racemase is transcriptionally induced by Abeta. Finally, the levels of serine racemase mRNA were elevated in Alzheimer's disease hippocampus, relative to age-matched controls. CONCLUSIONS: These data suggest that Abeta could contribute to neurodegeneration through stimulating microglia to release cooperative excitatory amino acids, including D-serine.
Figures
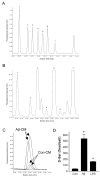

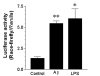
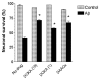
 0.005)] B. HAPI microglial cell line treated with 15 μM Aβ1–42. C. N9 microglial cell line treated with 300 ng/mL LPS or 10 nM sAPPα695.
0.005)] B. HAPI microglial cell line treated with 15 μM Aβ1–42. C. N9 microglial cell line treated with 300 ng/mL LPS or 10 nM sAPPα695.


Similar articles
-
Induction of serine racemase expression and D-serine release from microglia by secreted amyloid precursor protein (sAPP).Curr Alzheimer Res. 2007 Jul;4(3):243-51. doi: 10.2174/156720507781077241. Curr Alzheimer Res. 2007. PMID: 17627481
-
Induction of serine racemase by inflammatory stimuli is dependent on AP-1.Ann N Y Acad Sci. 2004 Dec;1035:133-46. doi: 10.1196/annals.1332.009. Ann N Y Acad Sci. 2004. PMID: 15681805
-
Small Aβ1-42 oligomer-induced membrane depolarization of neuronal and microglial cells: role of N-methyl-D-aspartate receptors.J Neurosci Res. 2015 Mar;93(3):475-86. doi: 10.1002/jnr.23510. Epub 2014 Nov 14. J Neurosci Res. 2015. PMID: 25400096
-
D-amino acids in the brain: the biochemistry of brain serine racemase.FEBS J. 2008 Jul;275(14):3538-45. doi: 10.1111/j.1742-4658.2008.06517.x. FEBS J. 2008. PMID: 18564178 Review.
-
The Energy Landscape of Human Serine Racemase.Front Mol Biosci. 2019 Jan 9;5:112. doi: 10.3389/fmolb.2018.00112. eCollection 2018. Front Mol Biosci. 2019. PMID: 30687716 Free PMC article. Review.
Cited by
-
Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate.J Neurosci. 2010 Apr 14;30(15):5346-56. doi: 10.1523/JNEUROSCI.5966-09.2010. J Neurosci. 2010. PMID: 20392956 Free PMC article.
-
Susceptibility to Calcium Dysregulation during Brain Aging.Front Aging Neurosci. 2009 Nov 27;1:2. doi: 10.3389/neuro.24.002.2009. eCollection 2009. Front Aging Neurosci. 2009. PMID: 20552053 Free PMC article.
-
MKP-1 reduces Aβ generation and alleviates cognitive impairments in Alzheimer's disease models.Signal Transduct Target Ther. 2019 Dec 6;4:58. doi: 10.1038/s41392-019-0091-4. eCollection 2019. Signal Transduct Target Ther. 2019. PMID: 31840000 Free PMC article.
-
Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice.Learn Mem. 2008 Dec 30;16(1):28-37. doi: 10.1101/lm.1112209. Print 2009 Jan. Learn Mem. 2008. PMID: 19117914 Free PMC article.
-
The role of D-serine as co-agonist of NMDA receptors in the nucleus accumbens: relevance to cocaine addiction.Front Synaptic Neurosci. 2014 Jul 16;6:16. doi: 10.3389/fnsyn.2014.00016. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25076900 Free PMC article. Review.
References
-
- Jansen KL, Faull RL, Dragunow M, Synek BL. Alzheimer's disease: changes in hippocampal N-methyl-D-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors--an autoradiographic study. Neuroscience. 1990;39:613–627. doi: 10.1016/0306-4522(90)90246-Z. - DOI - PubMed
-
- Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–4698. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

