Preserved fronto-striatal plasticity and enhanced procedural learning in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe
- PMID: 15286183
- PMCID: PMC498330
- DOI: 10.1101/lm.80604
Preserved fronto-striatal plasticity and enhanced procedural learning in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe
Abstract
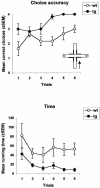
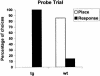
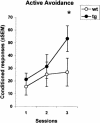
Mutations in the amyloid precursor protein (APP) gene inducing abnormal processing and deposition of beta-amyloid protein in the brain have been implicated in the pathogenesis of Alzheimer's disease (AD). Although Tg2576 mice with the Swedish mutation (hAPPswe) exhibit age-related Abeta-plaque formation in brain regions like the hippocampus, the amygdala, and the cortex, these mice show a rather specific deficit in hippocampal-dependent learning and memory tasks. In view of recent findings showing that neural systems subserving different forms of learning are not simply independent but that depressing or enhancing one system affects learning in another system, we decided to investigate fronto-striatal synaptic plasticity and related procedural learning in these mutants. Fronto-striatal long-term depression (LTD) induced by tetanic stimulation of the cortico-striatal input was similar in Tg2576 and wild-type control mice. Behavioral data, however, pointed to an enhancement of procedural learning in the mutants that showed robust motor-based learning in the cross maze and higher active avoidance scores. Thus, in this mouse model of AD, an intact striatal function associated with an impaired hippocampal function seems to provide neural conditions favorable to procedural learning. Our results suggest that focusing on preserved or enhanced forms of learning in AD patients might be of interest to describe the functional reorganization of the brain when one memory system is selectively compromised by neurological disease.
Figures
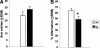
Similar articles
-
Progressive cognitive decline in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe.Genes Brain Behav. 2006 Apr;5(3):249-56. doi: 10.1111/j.1601-183X.2005.00160.x. Genes Brain Behav. 2006. PMID: 16594978
-
Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice.Neuroscience. 2015 Jul 9;298:357-66. doi: 10.1016/j.neuroscience.2015.04.038. Epub 2015 Apr 23. Neuroscience. 2015. PMID: 25917310
-
Soluble Aβ levels correlate with cognitive deficits in the 12-month-old APPswe/PS1dE9 mouse model of Alzheimer's disease.Behav Brain Res. 2011 Sep 23;222(2):342-50. doi: 10.1016/j.bbr.2011.03.072. Epub 2011 Apr 14. Behav Brain Res. 2011. PMID: 21513747
-
Presenilins and APP in neuritic and synaptic plasticity: implications for the pathogenesis of Alzheimer's disease.Neuromolecular Med. 2002;2(2):167-96. doi: 10.1385/NMM:2:2:167. Neuromolecular Med. 2002. PMID: 12428810 Review.
-
Synaptic structure and function in transgenic APP mice.Ann N Y Acad Sci. 2000;924:39-41. doi: 10.1111/j.1749-6632.2000.tb05558.x. Ann N Y Acad Sci. 2000. PMID: 11193800 Review.
Cited by
-
Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency.Nat Neurosci. 2014 Feb;17(2):223-31. doi: 10.1038/nn.3618. Epub 2014 Jan 19. Nat Neurosci. 2014. PMID: 24441681
-
Impaired hippocampus-dependent and facilitated striatum-dependent behaviors in mice lacking the δ opioid receptor.Neuropsychopharmacology. 2013 May;38(6):1050-9. doi: 10.1038/npp.2013.1. Epub 2013 Jan 4. Neuropsychopharmacology. 2013. PMID: 23303070 Free PMC article.
-
The role of beta-amyloid protein in synaptic function: implications for Alzheimer's disease therapy.Curr Neuropharmacol. 2006 Apr;4(2):149-63. doi: 10.2174/157015906776359531. Curr Neuropharmacol. 2006. PMID: 18615129 Free PMC article.
-
Tissue-Plasminogen Activator Attenuates Alzheimer's Disease-Related Pathology Development in APPswe/PS1 Mice.Neuropsychopharmacology. 2016 Apr;41(5):1297-307. doi: 10.1038/npp.2015.279. Epub 2015 Sep 9. Neuropsychopharmacology. 2016. PMID: 26349911 Free PMC article.
-
Place vs. Response Learning: History, Controversy, and Neurobiology.Front Behav Neurosci. 2021 Feb 11;14:598570. doi: 10.3389/fnbeh.2020.598570. eCollection 2020. Front Behav Neurosci. 2021. PMID: 33643005 Free PMC article.
References
-
- Ammassari-Teule, M., Middei, S., Passino, E., and Restivo, L. 2002. Enhanced procedural learning following β-amyloid protein (1-42) infusion in the rat. NeuroReport 13: 1679-1682. - PubMed
-
- Anderson, W.W. and Collingridge, G.L. 2001. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods 108: 71-83. - PubMed
-
- Ashe, K.H. 2001. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn. Mem. 8: 301-308. - PubMed
-
- Benzing, W.C., Wujek, J.R., Ward, E.K., Shaffer, D., Ashe, K.H., Younkin, S.G., and Brunden, K.R. 1999. Evidence for glial mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging 20: 581-589. - PubMed
-
- Bovet, D., Bovet-Nitti, F., and Oliverio, A. 1969. Genetic aspects of learning and memory in mice. Science 163: 139-149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
