Resolution of organelle docking and fusion kinetics in a cell-free assay
- PMID: 15286284
- PMCID: PMC511018
- DOI: 10.1073/pnas.0404583101
Resolution of organelle docking and fusion kinetics in a cell-free assay
Abstract
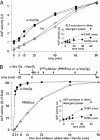
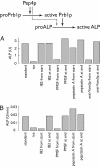
In vitro assays of compartment mixing have been key tools in the biochemical dissection of organelle docking and fusion. Many such assays measure compartment mixing through the enzymatic modification of reporter proteins. Homotypic fusion of yeast vacuoles is measured with a coupled assay of proteolytic maturation of pro-alkaline phosphatase (pro-ALP). A kinetic lag is observed between the end of docking, marked by the acquisition of resistance to anti-SNARE reagents, and ALP maturation. We therefore asked whether the time taken for pro-ALP maturation adds a kinetic lag to the measured fusion signal. Prb1p promotes ALP maturation; overproduction of Prb1p accelerates ALP activation in detergent lysates but does not alter the measured kinetics of docking or fusion. Thus, the lag between docking and ALP activation reflects a lag between docking and fusion. Many vacuoles in the population undergo multiple rounds of fusion; methods are presented for distinguishing the first round of fusion from ongoing rounds of fusion. A simple kinetic model distinguishes between two rates, the rate of fusion and the rate at which fusion competence is lost, and allows estimation of the number of rounds of fusion completed.
Figures


Similar articles
-
Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13010-5. doi: 10.1073/pnas.0700970104. Epub 2007 Jul 30. Proc Natl Acad Sci U S A. 2007. PMID: 17664431 Free PMC article.
-
A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion.J Cell Biol. 1998 Jan 12;140(1):61-9. doi: 10.1083/jcb.140.1.61. J Cell Biol. 1998. PMID: 9425154 Free PMC article.
-
Homotypic vacuolar fusion mediated by t- and v-SNAREs.Nature. 1997 May 8;387(6629):199-202. doi: 10.1038/387199a0. Nature. 1997. PMID: 9144293
-
Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms.Annu Rev Biochem. 2000;69:247-75. doi: 10.1146/annurev.biochem.69.1.247. Annu Rev Biochem. 2000. PMID: 10966459 Review.
-
Multiple sorting pathways between the late Golgi and the vacuole in yeast.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):211-30. doi: 10.1016/s0167-4889(98)00058-5. Biochim Biophys Acta. 1998. PMID: 9714809 Review.
Cited by
-
Vacuolar hydrolysis and efflux: current knowledge and unanswered questions.Autophagy. 2019 Feb;15(2):212-227. doi: 10.1080/15548627.2018.1545821. Epub 2018 Nov 22. Autophagy. 2019. PMID: 30422029 Free PMC article. Review.
-
Capture and release of partially zipped trans-SNARE complexes on intact organelles.J Cell Biol. 2009 May 4;185(3):535-49. doi: 10.1083/jcb.200811082. J Cell Biol. 2009. PMID: 19414611 Free PMC article.
-
LegC3, an effector protein from Legionella pneumophila, inhibits homotypic yeast vacuole fusion in vivo and in vitro.PLoS One. 2013;8(2):e56798. doi: 10.1371/journal.pone.0056798. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437241 Free PMC article.
-
Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13010-5. doi: 10.1073/pnas.0700970104. Epub 2007 Jul 30. Proc Natl Acad Sci U S A. 2007. PMID: 17664431 Free PMC article.
-
Cdc42p is activated during vacuole membrane fusion in a sterol-dependent subreaction of priming.J Biol Chem. 2010 Feb 12;285(7):4298-306. doi: 10.1074/jbc.M109.074609. Epub 2009 Dec 10. J Biol Chem. 2010. PMID: 20007700 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

