Understanding in vivo benzenoid metabolism in petunia petal tissue
- PMID: 15286288
- PMCID: PMC520771
- DOI: 10.1104/pp.104.045468
Understanding in vivo benzenoid metabolism in petunia petal tissue
Abstract
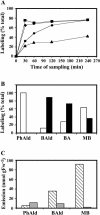
In vivo stable isotope labeling and computer-assisted metabolic flux analysis were used to investigate the metabolic pathways in petunia (Petunia hybrida) cv Mitchell leading from Phe to benzenoid compounds, a process that requires the shortening of the side chain by a C(2) unit. Deuterium-labeled Phe ((2)H(5)-Phe) was supplied to excised petunia petals. The intracellular pools of benzenoid/phenylpropanoid-related compounds (intermediates and end products) as well as volatile end products within the floral bouquet were analyzed for pool sizes and labeling kinetics by gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry. Modeling of the benzenoid network revealed that both the CoA-dependent, beta-oxidative and CoA-independent, non-beta-oxidative pathways contribute to the formation of benzenoid compounds in petunia flowers. The flux through the CoA-independent, non-beta-oxidative pathway with benzaldehyde as a key intermediate was estimated to be about 2 times higher than the flux through the CoA-dependent, beta-oxidative pathway. Modeling of (2)H(5)-Phe labeling data predicted that in addition to benzaldehyde, benzylbenzoate is an intermediate between l-Phe and benzoic acid. Benzylbenzoate is the result of benzoylation of benzyl alcohol, for which activity was detected in petunia petals. A cDNA encoding a benzoyl-CoA:benzyl alcohol/phenylethanol benzoyltransferase was isolated from petunia cv Mitchell using a functional genomic approach. Biochemical characterization of a purified recombinant benzoyl-CoA:benzyl alcohol/phenylethanol benzoyltransferase protein showed that it can produce benzylbenzoate and phenylethyl benzoate, both present in petunia corollas, with similar catalytic efficiencies.
Figures



References
-
- Ahmed MA, El-Mawla A, Beerhues L (2002) Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta 214: 727–733 - PubMed
-
- Alibert G, Ranjeva R (1971) Research on the enzymes catalyzing the biosynthesis of phenolic acids in Quercus pedunculata (Ehrh.): I – Formation of the first members of the cinnamic series and benzoic series. FEBS Lett 19: 11–14 - PubMed
-
- Anterola AM, Jeon JH, Davin LB, Lewis NG (2002) Transcriptional control of monolignol biosynthesis in Pinus taeda: factors affecting monolignol ratios and carbon allocation in phenylpropanoid metabolism. J Biol Chem 277: 18272–18280 - PubMed
-
- Appert C, Zon J, Amrhein N (2003) Kinetic analysis of the inhibition of phenylalanine ammonia-lyase by 2-aminoindan-2-phosphonic acid and other phenylalanine analogues. Phytochemistry 62: 415–422 - PubMed
-
- Bailey JE (1998) Mathematical modeling and analysis in biochemical engineering: past accomplishments and future opportunities. Biotechnol Prog 14: 8–20 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

