Fibrotic disease and the T(H)1/T(H)2 paradigm
- PMID: 15286725
- PMCID: PMC2702150
- DOI: 10.1038/nri1412
Fibrotic disease and the T(H)1/T(H)2 paradigm
Abstract
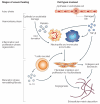
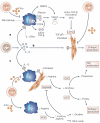
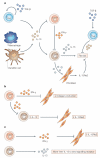
Tissue fibrosis (scarring) is a leading cause of morbidity and mortality. Current treatments for fibrotic disorders, such as idiopathic pulmonary fibrosis, hepatic fibrosis and systemic sclerosis, target the inflammatory cascade, but they have been widely unsuccessful, largely because the mechanisms that are involved in fibrogenesis are now known to be distinct from those involved in inflammation. Several experimental models have recently been developed to dissect the molecular mechanisms of wound healing and fibrosis. It is hoped that by better understanding the immunological mechanisms that initiate, sustain and suppress the fibrotic process, we will achieve the elusive goal of targeted and effective therapeutics for fibroproliferative diseases.
Figures

References
-
- Cotran RS, Kumar V, Collins T. In: Pathologic Basis of Disease. Cotran RS, Kumar V, Collins T, editors. Vol. 6. W. B. Saunders Company; Philadelphia: 1999. pp. 89–112.
-
- Wynn TA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. An early example of the opposing activities of TH1 and TH2 responses in a progressive fibrotic disease. - PubMed
-
- Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000;164:6406–6416. - PubMed
-
- Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon-γ in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp. Lung Res. 1995;21:791–808. - PubMed
-
- Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L92–L97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

