Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia
- PMID: 15286798
- PMCID: PMC484981
- DOI: 10.1172/JCI21153
Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia
Abstract
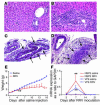
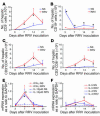
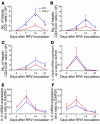
The etiology and pathogenesis of bile duct obstruction in children with biliary atresia are largely unknown. We have previously reported that, despite phenotypic heterogeneity, genomic signatures of livers from patients display a proinflammatory phenotype. Here, we address the hypothesis that production of IFN-gamma is a key pathogenic mechanism of disease using a mouse model of rotavirus-induced biliary atresia. We found that rotavirus infection of neonatal mice has a unique tropism to bile duct cells, and it triggers a hepatobiliary inflammation by IFN-gamma-producing CD4(+) and CD8(+) lymphocytes. The inflammation is tissue specific, resulting in progressive jaundice, growth failure, and greater than 90% mortality due to obstruction of extrahepatic bile ducts. In this model, the genetic loss of IFN-gamma did not alter the onset of jaundice, but it remarkably suppressed the tissue-specific targeting of T lymphocytes and completely prevented the inflammatory and fibrosing obstruction of extrahepatic bile ducts. As a consequence, jaundice resolved, and long-term survival improved to greater than 80%. Notably, administration of recombinant IFN-gamma led to recurrence of bile duct obstruction following rotavirus infection of IFN-gamma-deficient mice. Thus, IFN-gamma-driven obstruction of bile ducts is a key pathogenic mechanism of disease and may constitute a therapeutic target to block disease progression in patients with biliary atresia.
Figures

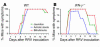



References
-
- Balistreri WF, et al. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. - PubMed
-
- Perlmutter DH, Shepherd RW. Extrahepatic biliary atresia: a disease or a phenotype? Hepatology. 2002;35:1297–1304. - PubMed
-
- Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J. Pediatr. Gastroenterol. Nutr. 2003;37:4–21. - PubMed
-
- Bezerra JA, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1563–1659. - PubMed
-
- Riepenhoff-Talty M, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 1993;33:394–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

