Central and peripheral actions of somatostatin on the growth hormone-IGF-I axis
- PMID: 15286801
- PMCID: PMC484973
- DOI: 10.1172/JCI19933
Central and peripheral actions of somatostatin on the growth hormone-IGF-I axis
Abstract
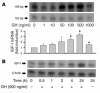
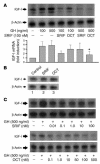
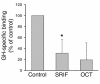
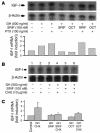
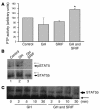
Somatostatin (SRIF) analogs provide safe and effective therapy for acromegaly. In a proportion of patients, however, SRIF analogs may lead to discordant growth hormone (GH) and IGF-I suppression, which suggests a more complex mechanism than attributable to inhibition of GH release alone. To elucidate whether SRIF acts peripherally on the GH-IGF-I axis, we showed that rat hepatocytes express somatostatin receptor subtypes-2 and -3 and that IGF-I mRNA and protein levels were suppressed in a dose-dependent manner by administration of octreotide. The inhibitory effect of SRIF was not apparent without added GH and in the presence of GH was specific for IGF-I induction and did not inhibit GH-induced c-myc or extracellular signal regulated kinase (ERK) phosphorylation. Pertussis toxin treatment of hepatocytes incubated with GH and SRIF, or with GH and octreotide, abrogated the inhibitory effect on GH-induced IGF-I, which confirms the requirement for the inhibitory G-protein. Treatment with SRIF and GH increased protein tyrosine phosphatase (PTP) activity and inhibited signal transducer and activator of transcription-5b (STAT5b) phosphorylation and nuclear localization. Octreotide also inhibited GH-stimulated IGF-I protein content of ex vivo-perfused rat livers. The results demonstrate that SRIF acts both centrally and peripherally to control the GH-IGF-I axis, providing a mechanistic explanation for SRIF analog action in treating patients with GH-secreting pituitary adenomas.
Figures
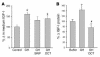

Similar articles
-
In vitro effects of somatostatin on the growth hormone-insulin-like growth factor axis in orange-spotted grouper (Epinephelus coioides).Gen Comp Endocrinol. 2016 Oct 1;237:1-9. doi: 10.1016/j.ygcen.2015.10.014. Epub 2015 Oct 23. Gen Comp Endocrinol. 2016. PMID: 26526981
-
SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile.Eur J Endocrinol. 2002 May;146(5):707-16. doi: 10.1530/eje.0.1460707. Eur J Endocrinol. 2002. PMID: 11980628
-
SOM230: a new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs.Endocrinology. 2002 Oct;143(10):4123-30. doi: 10.1210/en.2002-220219. Endocrinology. 2002. PMID: 12239124
-
Somatostatin analogs for diagnosis and treatment of cancer.Pharmacol Ther. 1993 Nov;60(2):245-64. doi: 10.1016/0163-7258(93)90009-3. Pharmacol Ther. 1993. PMID: 7912834 Review.
-
Somatostatin receptors: from signaling to clinical practice.Front Neuroendocrinol. 2013 Aug;34(3):228-52. doi: 10.1016/j.yfrne.2013.07.005. Epub 2013 Jul 18. Front Neuroendocrinol. 2013. PMID: 23872332 Review.
Cited by
-
Management of persistent acromegaly following primary therapy: The current landscape in the UK.Endocrinol Diabetes Metab. 2020 Jun 9;3(3):e00158. doi: 10.1002/edm2.158. eCollection 2020 Jul. Endocrinol Diabetes Metab. 2020. PMID: 32704572 Free PMC article. Review.
-
Somatostatin-Dopamine Chimeric Molecules in Neuroendocrine Neoplasms.J Clin Med. 2021 Feb 1;10(3):501. doi: 10.3390/jcm10030501. J Clin Med. 2021. PMID: 33535394 Free PMC article. Review.
-
Noncanonical suppression of GH-dependent isoforms of cytochrome P450 by the somatostatin analog octreotide.J Endocrinol. 2013 Jan 2;216(1):87-97. doi: 10.1530/JOE-12-0255. Print 2013 Jan. J Endocrinol. 2013. PMID: 23077183 Free PMC article.
-
Somatostatin Receptors and Analogs in Pheochromocytoma and Paraganglioma: Old Players in a New Precision Medicine World.Front Endocrinol (Lausanne). 2021 Mar 29;12:625312. doi: 10.3389/fendo.2021.625312. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33854479 Free PMC article. Review.
-
The GH/IGF-1 Axis and Heart Failure.Curr Cardiol Rev. 2009 Aug;5(3):203-15. doi: 10.2174/157340309788970306. Curr Cardiol Rev. 2009. PMID: 20676279 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

