Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone
- PMID: 15286802
- PMCID: PMC484982
- DOI: 10.1172/JCI21620
Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone
Abstract
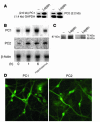
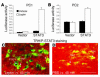
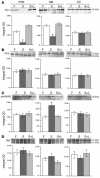
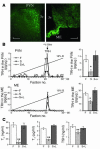
Regulation of energy balance by leptin involves regulation of several neuropeptides, including thyrotropin-releasing hormone (TRH). Synthesized from a larger inactive precursor, its maturation requires proteolytic cleavage by prohormone convertases 1 and 2 (PC1 and PC2). Since this maturation in response to leptin requires prohormone processing, we hypothesized that leptin might regulate hypothalamic PC1 and PC2 expression, ultimately leading to coordinated processing of prohormones into mature peptides. Using hypothalamic neurons, we found that leptin stimulated PC1 and PC2 mRNA and protein expression and also increased PC1 and PC2 promoter activities in transfected 293T cells. Starvation of rats, leading to low serum leptin levels, decreased PC1 and PC2 gene and protein expression in the paraventricular nucleus (PVN) of the hypothalamus. Exogenous administration of leptin to fasted animals restored PC1 levels in the median eminence (ME) and the PVN to approximately the level found in fed control animals. Consistent with this regulation of PCs in the PVN, concentrations of TRH in the PVN and ME were substantially reduced in the fasted animals relative to the fed animals, and leptin reversed this decrease. Further analysis showed that proteolytic cleavage of pro-thyrotropin-releasing hormone (proTRH) at known PC cleavage sites was reduced by fasting and increased in animals given leptin. Combined, these findings suggest that leptin-dependent stimulation of hypothalamic TRH expression involves both activation of trh transcription and stimulation of PC1 and PC2 expression, which lead to enhanced processing of proTRH into mature TRH.
Figures




Similar articles
-
Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin.Endocrinology. 2007 Sep;148(9):4191-200. doi: 10.1210/en.2007-0173. Epub 2007 Jun 21. Endocrinology. 2007. PMID: 17584972 Review.
-
Cellular colocalization and coregulation between hypothalamic pro-TRH and prohormone convertases in hypothyroidism.Am J Physiol Endocrinol Metab. 2007 Jan;292(1):E175-86. doi: 10.1152/ajpendo.00288.2006. Epub 2006 Aug 22. Am J Physiol Endocrinol Metab. 2007. PMID: 16926379
-
Biosynthesis of proTRH-derived peptides in prohormone convertase 1 and 2 knockout mice.Peptides. 2012 May;35(1):42-8. doi: 10.1016/j.peptides.2012.02.024. Epub 2012 Mar 6. Peptides. 2012. PMID: 22421509
-
Identification of the thyrotropin-releasing hormone precursor, its processing products, and its coexpression with convertase 1 in primary cultures of hypothalamic neurons: anatomic distribution of PC1 and PC2.Endocrinology. 1996 Dec;137(12):5651-61. doi: 10.1210/endo.137.12.8940396. Endocrinology. 1996. PMID: 8940396
-
The biosynthesis and processing of neuropeptides: lessons from prothyrotropin releasing hormone (proTRH).Front Biosci. 2007 May 1;12:3554-65. doi: 10.2741/2334. Front Biosci. 2007. PMID: 17485321 Review.
Cited by
-
NEW INSIGHTS INTO THE HYPOTHALAMIC-PITUITARY-THYROID AXIS.Acta Endocrinol (Buchar). 2016 Apr-Jun;12(2):125-129. doi: 10.4183/aeb.2016.125. Acta Endocrinol (Buchar). 2016. PMID: 31149076 Free PMC article.
-
Central Sirt1 regulates body weight and energy expenditure along with the POMC-derived peptide α-MSH and the processing enzyme CPE production in diet-induced obese male rats.Endocrinology. 2015 Mar;156(3):961-74. doi: 10.1210/en.2014-1970. Epub 2014 Dec 30. Endocrinology. 2015. PMID: 25549049 Free PMC article.
-
Leptin as a modulator of neuroendocrine function in humans.Yonsei Med J. 2012 Jul 1;53(4):671-9. doi: 10.3349/ymj.2012.53.4.671. Yonsei Med J. 2012. PMID: 22665330 Free PMC article. Review.
-
Neurotensin via Type I Receptor Modulates the Endotoxemia Induced Oxido-Inflammatory Stress on the Sympathetic Adrenomedullary System of Mice Regulating NF-κβ/Nor-Epinephrine Pathway.Cell Biochem Biophys. 2025 Sep;83(3):2961-2975. doi: 10.1007/s12013-025-01679-5. Epub 2025 Jan 29. Cell Biochem Biophys. 2025. PMID: 39881060
-
The role of thyroid function in female and male infertility: a narrative review.J Endocrinol Invest. 2023 Jan;46(1):15-26. doi: 10.1007/s40618-022-01883-7. Epub 2022 Aug 9. J Endocrinol Invest. 2023. PMID: 35945393 Free PMC article. Review.
References
-
- Seidah NG, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr. Opin. Biotechnol. 1997;8:602–607. - PubMed
-
- Steiner DF. The proprotein convertases. Curr. Opin. Chem. Biol. 1998;2:31–39. - PubMed
-
- Fricker L. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–321. - PubMed
-
- Dong W, Fricker LD, Day R. Carboxypeptidase D is a potential candidate to carry out redundant processing functions of carboxypeptidase E based on comparative distribution studies in the rat central nervous system. Neuroscience. 1999;89:1301–1317. - PubMed
-
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide α-amidation. Annu. Rev. Neurosci. 1992;15:57–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

