Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects
- PMID: 15286804
- PMCID: PMC489961
- DOI: 10.1172/JCI21102
Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects
Abstract
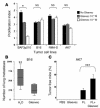
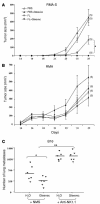
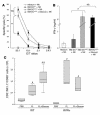
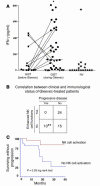
Mutant isoforms of the KIT or PDGF receptors expressed by gastrointestinal stromal tumors (GISTs) are considered the therapeutic targets for STI571 (imatinib mesylate; Gleevec), a specific inhibitor of these tyrosine kinase receptors. Case reports of clinical efficacy of Gleevec in GISTs lacking the typical receptor mutations prompted a search for an alternate mode of action. Here we show that Gleevec can act on host DCs to promote NK cell activation. DC-mediated NK cell activation was triggered in vitro and in vivo by treatment of DCs with Gleevec as well as by a loss-of-function mutation of KIT. Therefore, tumors that are refractory to the antiproliferative effects of Gleevec in vitro responded to Gleevec in vivo in an NK cell-dependent manner. Longitudinal studies of Gleevec-treated GIST patients revealed a therapy-induced increase in IFN-gamma production by NK cells, correlating with an enhanced antitumor response. These data point to a novel mode of antitumor action for Gleevec.
Figures


References
-
- Rubin BP, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. - PubMed
-
- Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;31:708–710. - PubMed
-
- Apperley JF, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N. Engl. J. Med. 2002;347:481–487. - PubMed
-
- Buchdunger E, O’Reilly T, Wood J. Pharmacology of imatinib (STI571) Eur. J. Cancer. 2002;38:S28–S36. - PubMed
-
- Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 2002;20:1692–1703. - PubMed

