Bone marrow-derived immune cells regulate vascular disease through a p27(Kip1)-dependent mechanism
- PMID: 15286808
- PMCID: PMC484975
- DOI: 10.1172/JCI20176
Bone marrow-derived immune cells regulate vascular disease through a p27(Kip1)-dependent mechanism
Abstract
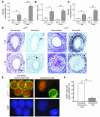
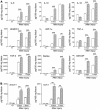
The cyclin-dependent kinase inhibitors are key regulators of cell cycle progression. Although implicated in carcinogenesis, they inhibit the proliferation of a variety of normal cell types, and their role in diverse human diseases is not fully understood. Here, we report that p27(Kip1) plays a major role in cardiovascular disease through its effects on the proliferation of bone marrow-derived (BM-derived) immune cells that migrate into vascular lesions. Lesion formation after mechanical arterial injury was markedly increased in mice with homozygous deletion of p27(Kip1), characterized by prominent vascular infiltration by immune and inflammatory cells. Vascular occlusion was substantially increased when BM-derived cells from p27(-/-) mice repopulated vascular lesions induced by mechanical injury in p27(+/+) recipients, in contrast to p27(+/+) BM donors. To determine the contribution of immune cells to vascular injury, transplantation was performed with BM derived from RAG(-/-) and RAG(+/+) mice. RAG(+/+) BM markedly exacerbated vascular proliferative lesions compared with what was found in RAG(-/-) donors. Taken together, these findings suggest that vascular repair and regeneration is regulated by the proliferation of BM-derived hematopoietic and nonhematopoietic cells through a p27(Kip1)-dependent mechanism and that immune cells largely mediate these effects.
Figures



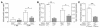
References
-
- Ridker PM, Rifai N, Rose L, Burning JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002;347:1557–1565. - PubMed
-
- Binder CJ, et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
-
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002;91:281–291. - PubMed
-
- Polyak K, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

